Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions
- PMID: 12719560
- PMCID: PMC154040
- DOI: 10.1128/jvi.77.10.5678-5684.2003
Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions
Abstract
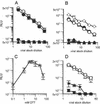
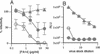
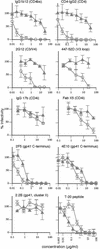
We previously described a human immunodeficiency virus type 1 (HIV-1) envelope mutant that introduces a disulfide bridge between the gp120 surface proteins and gp41 transmembrane proteins (J. M. Binley, R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore, J. Virol. 74:627-643, 2000). Here we produced pseudovirions bearing the mutant envelope and a reporter gene to examine the mutant's infectious properties. These pseudovirions attach to cells expressing CD4 and coreceptor but infect only when triggered with reducing agent, implying that gp120-gp41 dissociation is necessary for infection. Further studies suggested that virus entry was arrested after CD4 and coreceptor engagement. By measuring the activities of various entry inhibitors against the arrested intermediate, we found that gp120-targeting inhibitors typically act prior to virus attachment, whereas gp41 inhibitors are able to act postattachment. Unexpectedly, a significant fraction of antibodies in HIV-1-positive sera neutralized virus postattachment, suggesting that downstream fusion events and structures figure prominently in the host immune response. Overall, this disulfide-shackled virus is a unique tool with potential utility in vaccine design, drug discovery, and elucidation of the HIV-1 entry process.
Figures

Similar articles
-
Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits.J Virol. 2000 Jun;74(11):5091-100. doi: 10.1128/jvi.74.11.5091-5100.2000. J Virol. 2000. PMID: 10799583 Free PMC article.
-
Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion.J Virol. 2003 May;77(10):5829-36. doi: 10.1128/jvi.77.10.5829-5836.2003. J Virol. 2003. PMID: 12719576 Free PMC article.
-
Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1.J Virol. 2002 Sep;76(17):8875-89. doi: 10.1128/jvi.76.17.8875-8889.2002. J Virol. 2002. PMID: 12163607 Free PMC article.
-
Progress in targeting HIV-1 entry.Drug Discov Today. 2005 Aug 15;10(16):1085-94. doi: 10.1016/S1359-6446(05)03550-6. Drug Discov Today. 2005. PMID: 16182193 Review.
-
Recent advances in understanding the molecular mechanisms of HIV-1 entry and fusion: revisiting current targets and considering new options for therapeutic intervention.Curr HIV Res. 2004 Jul;2(3):223-34. doi: 10.2174/1570162043351327. Curr HIV Res. 2004. PMID: 15279586 Review.
Cited by
-
A nonparametric procedure for defining a new humoral immunologic profile in a pilot study on HIV infected patients.PLoS One. 2013;8(3):e58768. doi: 10.1371/journal.pone.0058768. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533590 Free PMC article.
-
Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies.J Virol. 2004 Dec;78(23):13232-52. doi: 10.1128/JVI.78.23.13232-13252.2004. J Virol. 2004. PMID: 15542675 Free PMC article.
-
Heterologous epitope-scaffold prime:boosting immuno-focuses B cell responses to the HIV-1 gp41 2F5 neutralization determinant.PLoS One. 2011 Jan 26;6(1):e16074. doi: 10.1371/journal.pone.0016074. PLoS One. 2011. PMID: 21297864 Free PMC article.
-
Antibody to gp41 MPER alters functional properties of HIV-1 Env without complete neutralization.PLoS Pathog. 2014 Jul 24;10(7):e1004271. doi: 10.1371/journal.ppat.1004271. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 25058619 Free PMC article.
-
Forced virus evolution reveals functional crosstalk between the disulfide bonded region and membrane proximal ectodomain region of HIV-1 gp41.Retrovirology. 2013 Apr 23;10:44. doi: 10.1186/1742-4690-10-44. Retrovirology. 2013. PMID: 23618462 Free PMC article.
References
-
- Binley, J. M., H. J. Ditzel, C. F. Barbas III, N. Sullivan, J. Sodroski, P. W. Parren, and D. R. Burton. 1996. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res. Hum. Retrovir. 12:911-924. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
-
- Burton, D. R. 2002. Opinion: Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2:706-713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

