Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer
- PMID: 12719475
- PMCID: PMC2172899
- DOI: 10.1083/jcb.200211097
Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer
Abstract
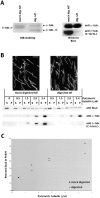
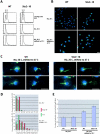
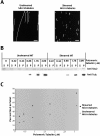
The Dis1/XMAP215 family of microtubule-associated proteins conserved from yeast to mammals is essential for cell division. XMAP215, the Xenopus member of this family, has been shown to stabilize microtubules in vitro, but other members of this family have not been biochemically characterized. Here we investigate the properties of the Saccharomyces cerevisiae homologue Stu2p in vitro. Surprisingly, Stu2p is a microtubule destabilizer that binds preferentially to microtubule plus ends. Quantitative analysis of microtubule dynamics suggests that Stu2p induces microtubule catastrophes by sterically interfering with tubulin addition to microtubule ends. These results reveal both a new biochemical activity for a Dis1/XMAP215 family member and a novel mechanism for microtubule destabilization.
Figures
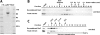



Similar articles
-
Stu2p binds tubulin and undergoes an open-to-closed conformational change.J Cell Biol. 2006 Mar 27;172(7):1009-22. doi: 10.1083/jcb.200511010. J Cell Biol. 2006. PMID: 16567500 Free PMC article.
-
The TOG protein Stu2/XMAP215 interacts covalently and noncovalently with SUMO.Cytoskeleton (Hoboken). 2018 Jul;75(7):290-306. doi: 10.1002/cm.21449. Epub 2018 Jul 2. Cytoskeleton (Hoboken). 2018. PMID: 29729126 Free PMC article.
-
Control of microtubule dynamics by Stu2p is essential for spindle orientation and metaphase chromosome alignment in yeast.Mol Biol Cell. 2001 Sep;12(9):2870-80. doi: 10.1091/mbc.12.9.2870. Mol Biol Cell. 2001. PMID: 11553724 Free PMC article.
-
MAPping the eukaryotic tree of life: structure, function, and evolution of the MAP215/Dis1 family of microtubule-associated proteins.Int Rev Cytol. 2004;239:179-272. doi: 10.1016/S0074-7696(04)39004-2. Int Rev Cytol. 2004. PMID: 15464854 Review.
-
Microtubule dynamics: new surprises from an old MAP.Curr Biol. 2003 Aug 5;13(15):R597-9. doi: 10.1016/s0960-9822(03)00524-4. Curr Biol. 2003. PMID: 12906811 Review.
Cited by
-
Multiparametric analysis of CLASP-interacting protein functions during interphase microtubule dynamics.Mol Cell Biol. 2013 Apr;33(8):1528-45. doi: 10.1128/MCB.01442-12. Epub 2013 Feb 4. Mol Cell Biol. 2013. PMID: 23382075 Free PMC article.
-
Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules.J Cell Biol. 2005 Sep 26;170(7):1039-46. doi: 10.1083/jcb.200504097. J Cell Biol. 2005. PMID: 16186253 Free PMC article.
-
Regulation of microtubule dynamics by Bim1 and Bik1, the budding yeast members of the EB1 and CLIP-170 families of plus-end tracking proteins.Mol Biol Cell. 2010 Jun 15;21(12):2013-23. doi: 10.1091/mbc.e10-02-0083. Epub 2010 Apr 14. Mol Biol Cell. 2010. PMID: 20392838 Free PMC article.
-
The XMAP215-family protein DdCP224 is required for cortical interactions of microtubules.BMC Cell Biol. 2004 Jun 8;5:24. doi: 10.1186/1471-2121-5-24. BMC Cell Biol. 2004. PMID: 15186508 Free PMC article.
-
Requirement for the budding yeast polo kinase Cdc5 in proper microtubule growth and dynamics.Eukaryot Cell. 2008 Mar;7(3):444-53. doi: 10.1128/EC.00283-07. Epub 2008 Jan 4. Eukaryot Cell. 2008. PMID: 18178775 Free PMC article.
References
-
- Akhmanova, A., C.C. Hoogenraad, K. Drabek, T. Stepanova, B. Dortland, T. Verkerk, W. Vermeulen, B.M. Burgering, C.I. De Zeeuw, F. Grosveld, and N. Galjart. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 104:923–935. - PubMed
-
- Ashford, A., S.S.L. Andersen, and A. Hyman. 1998. Preparation of tubulin from bovine brain. Cell Biology: A Laboratory Handbook. 2nd edition. Vol. 2. Julio E. Cells, editor. Academic Press, Inc., Orlando, FL. 205–212.
-
- Belmont, L., and T. Mitchison. 1996. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rates of microtubules. Cell. 84:623–631. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

