Acidic pH induces topoisomerase II-mediated DNA damage
- PMID: 12692309
- PMCID: PMC154323
- DOI: 10.1073/pnas.0935978100
Acidic pH induces topoisomerase II-mediated DNA damage
Abstract
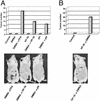
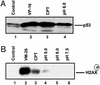
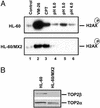
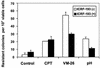
Acidic pH plays an important role in various pathophysiological states and has been demonstrated to be carcinogenic in animal models. Recent studies have also implicated acidic pH in the development of preneoplastic Barrett's esophagus in human. However, little is known about the molecular mechanism underlying acidic pH-induced carcinogenesis. In the current study, we show that acidic pH, like the topoisomerase II (TOP2) poison VP-16 (demethylepipodophyllotoxin ethylidene-beta-D-glucoside), induces tumors in 9,10-dimethyl-1,2-benzanthracene(DMBA)-initiated mice. The following studies in tissue culture models have suggested that acidic pH acts like a TOP2 poison to induce TOP2-mediated DNA damage: (i) acidic pH induces TOP2-dependent DNA damage signals as evidenced by up-regulation of p53 and Ser-139 phosphorylation of H2AX [a substrate for ataxia telangiectasia mutated (ATM)ATM and Rad3-related (ATR) kinases]; (ii) acidic pH-induced cytotoxicity in tumor cells is reduced in TOP2-deficient cells; (iii) acidic pH increases the mutation frequency of the hypoxanthine phosphoribosyl transferase (HPRT) gene in a TOP2-dependent manner; and (iv) acidic pH induces reversible TOP2-mediated DNA strand breaks in vitro. We discuss the possibility that TOP2-mediated DNA damage may contribute to acidic pH-induced carcinogenesis.
Figures

Similar articles
-
Topoisomerase II-mediated DNA cleavage and mutagenesis activated by nitric oxide underlie the inflammation-associated tumorigenesis.Antioxid Redox Signal. 2013 Apr 1;18(10):1129-40. doi: 10.1089/ars.2012.4620. Epub 2012 Nov 23. Antioxid Redox Signal. 2013. PMID: 22998676
-
The topoisomerase IIbeta circular clamp arrests transcription and signals a 26S proteasome pathway.Proc Natl Acad Sci U S A. 2003 Mar 18;100(6):3239-44. doi: 10.1073/pnas.0736401100. Epub 2003 Mar 10. Proc Natl Acad Sci U S A. 2003. PMID: 12629207 Free PMC article.
-
Ataxia telangiectasia mutated-dependent regulation of topoisomerase II alpha expression and sensitivity to topoisomerase II inhibitor.Cancer Sci. 2013 Feb;104(2):178-84. doi: 10.1111/cas.12067. Epub 2013 Jan 13. Cancer Sci. 2013. PMID: 23163762 Free PMC article.
-
Mechanisms to Repair Stalled Topoisomerase II-DNA Covalent Complexes.Mol Pharmacol. 2022 Jan;101(1):24-32. doi: 10.1124/molpharm.121.000374. Epub 2021 Oct 23. Mol Pharmacol. 2022. PMID: 34689119 Review.
-
Type II DNA Topoisomerases Cause Spontaneous Double-Strand Breaks in Genomic DNA.Genes (Basel). 2019 Oct 30;10(11):868. doi: 10.3390/genes10110868. Genes (Basel). 2019. PMID: 31671674 Free PMC article. Review.
Cited by
-
Serum Bicarbonate Concentration and Cause-Specific Mortality: The National Health and Nutrition Examination Survey 1999-2010.Mayo Clin Proc. 2020 Jan;95(1):113-123. doi: 10.1016/j.mayocp.2019.05.036. Epub 2019 Dec 4. Mayo Clin Proc. 2020. PMID: 31812253 Free PMC article.
-
On the Importance of Acidity in Cancer Cells and Therapy.Biology (Basel). 2024 Mar 29;13(4):225. doi: 10.3390/biology13040225. Biology (Basel). 2024. PMID: 38666837 Free PMC article. Review.
-
Decreased NHE3 expression in colon cancer is associated with DNA damage, increased inflammation and tumor growth.Sci Rep. 2022 Aug 30;12(1):14725. doi: 10.1038/s41598-022-19091-x. Sci Rep. 2022. PMID: 36042372 Free PMC article.
-
Unravelling the role of tumor microenvironment responsive nanobiomaterials in spatiotemporal controlled drug delivery for lung cancer therapy.Drug Deliv Transl Res. 2025 Feb;15(2):407-435. doi: 10.1007/s13346-024-01673-z. Epub 2024 Jul 22. Drug Deliv Transl Res. 2025. PMID: 39037533 Review.
-
Na+/H+-Exchanger Family as Novel Prognostic Biomarkers in Colorectal Cancer.J Oncol. 2021 Nov 1;2021:3241351. doi: 10.1155/2021/3241351. eCollection 2021. J Oncol. 2021. PMID: 34759967 Free PMC article.
References
-
- Williams A C, Collard T J, Paraskeva C. Oncogene. 1999;18:3199–3204. - PubMed
-
- Yamamoto D, Kiyozuka Y, Uemura Y, Yamamoto C, Takemoto H, Hirata H, Tanaka K, Hioki K, Tsubura A. J Cancer Res Clin Oncol. 2000;126:191–197. - PubMed
-
- Chevfec G, Schnell T, Sontag S. Am J Clin Pathol. 1992;98:5–7. - PubMed
-
- Shaheen N, Ransohoff D F. J Am Med Assoc. 2002;287:1972–1981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

