Intersectin 1L guanine nucleotide exchange activity is regulated by adjacent src homology 3 domains that are also involved in endocytosis
- PMID: 12686614
- PMCID: PMC153127
- DOI: 10.1091/mbc.e02-08-0494
Intersectin 1L guanine nucleotide exchange activity is regulated by adjacent src homology 3 domains that are also involved in endocytosis
Abstract
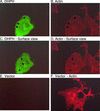
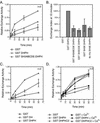
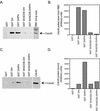
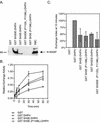
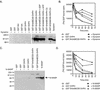
Intersectin 1L is a scaffolding protein involved in endocytosis that also has guanine nucleotide exchange activity for Cdc42. In the context of the full-length protein, the catalytic exchange activity of the DH domain is repressed. Here we use biochemical methods to dissect the mechanism for this inhibition. We demonstrate that the intersectin 1L SH3 domains, which bind endocytic proteins, directly inhibit the activity of the DH domain in assays for both binding and exchange of Cdc42. This inhibitory mechanism seems to act through steric hindrance of Cdc42 binding by an intramolecular interaction between the intersectin 1L SH3 domain region and the adjacent DH domain. Surprisingly, the mode of SH3 domain binding is other than through the proline peptide binding pocket. The dual role of the SH3 domains in endocytosis and repression of exchange activity suggests that the intersectin 1L exchange activity is regulated by endocytosis. We show that the endocytic protein, dynamin, competes for binding to the SH3 domains with the neural Wiskott-Aldrich Syndrome protein, an actin filament nucleation protein that is a substrate for activated Cdc42. Swapping of SH3 domain binding partners might act as a switch controlling the actin nucleation activity of intersectin 1L.
Figures


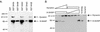
Similar articles
-
Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP.Nat Cell Biol. 2001 Oct;3(10):927-32. doi: 10.1038/ncb1001-927. Nat Cell Biol. 2001. PMID: 11584276
-
The minimal autoinhibited unit of the guanine nucleotide exchange factor intersectin.PLoS One. 2010 Jun 24;5(6):e11291. doi: 10.1371/journal.pone.0011291. PLoS One. 2010. PMID: 20585582 Free PMC article.
-
Intersectin-1L nucleotide exchange factor regulates secretory granule exocytosis by activating Cdc42.EMBO J. 2006 Aug 9;25(15):3494-503. doi: 10.1038/sj.emboj.7601247. Epub 2006 Jul 27. EMBO J. 2006. PMID: 16874303 Free PMC article.
-
Ever-expanding network of dynamin-interacting proteins.Mol Neurobiol. 2006 Oct;34(2):129-36. doi: 10.1385/MN:34:2:129. Mol Neurobiol. 2006. PMID: 17220534 Review.
-
Regulatory role of SH3 domain-mediated protein-protein interactions in synaptic vesicle endocytosis.Cell Signal. 1999 Apr;11(4):229-38. doi: 10.1016/s0898-6568(98)00059-x. Cell Signal. 1999. PMID: 10372800 Review.
Cited by
-
Intersectin 1 enhances Cbl ubiquitylation of epidermal growth factor receptor through regulation of Sprouty2-Cbl interaction.Mol Cell Biol. 2012 Feb;32(4):817-25. doi: 10.1128/MCB.05647-11. Epub 2011 Dec 12. Mol Cell Biol. 2012. PMID: 22158968 Free PMC article.
-
Role of numb in dendritic spine development with a Cdc42 GEF intersectin and EphB2.Mol Biol Cell. 2006 Mar;17(3):1273-85. doi: 10.1091/mbc.e05-07-0700. Epub 2006 Jan 4. Mol Biol Cell. 2006. PMID: 16394100 Free PMC article.
-
Control of synapse development and plasticity by Rho GTPase regulatory proteins.Prog Neurobiol. 2011 Jul;94(2):133-48. doi: 10.1016/j.pneurobio.2011.04.011. Epub 2011 Apr 22. Prog Neurobiol. 2011. PMID: 21530608 Free PMC article. Review.
-
Emerging roles for intersectin (ITSN) in regulating signaling and disease pathways.Int J Mol Sci. 2013 Apr 10;14(4):7829-52. doi: 10.3390/ijms14047829. Int J Mol Sci. 2013. PMID: 23574942 Free PMC article. Review.
-
Inhibition of Cdc42-intersectin interaction by small molecule ZCL367 impedes cancer cell cycle progression, proliferation, migration, and tumor growth.Cancer Biol Ther. 2019;20(6):740-749. doi: 10.1080/15384047.2018.1564559. Epub 2019 Mar 8. Cancer Biol Ther. 2019. PMID: 30849276 Free PMC article.
References
-
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. - PubMed
-
- Bustelo XR. Regulation of Vav proteins by intramolecular events. Front Biosci. 2002;7:d24–d30. - PubMed
-
- Cicchetti P, Mayer BJ, Thiel G, Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992;257:803–806. - PubMed
-
- Clark SG, Stern MJ, Horvitz HR. C. elegans cell-signaling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. - PubMed
-
- Clift-O'Grady L, Desnos C, Lichtenstein Y, Faundez V, Horng JT, Kelly RB. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods. 1998;16:150–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

