Effects of the eukaryotic pore-forming cytolysin Equinatoxin II on lipid membranes and the role of sphingomyelin
- PMID: 12668447
- PMCID: PMC1302805
- DOI: 10.1016/S0006-3495(03)75044-9
Effects of the eukaryotic pore-forming cytolysin Equinatoxin II on lipid membranes and the role of sphingomyelin
Abstract
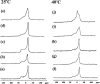
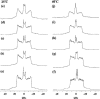
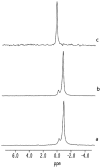
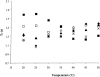
Equinatoxin II (EqtII), a protein toxin from the sea anemone Actinia equina, readily creates pores in sphingomyelin-containing lipid membranes. The perturbation by EqtII of model lipid membranes composed of dimyristoylphosphatidycholine and sphingomyelin (10 mol %) was investigated using wideline phosphorus-31 and deuterium NMR. The preferential interaction between EqtII (0.1 and 0.4 mol %) and the individual bilayer lipids was studied by (31)P magic angle spinning NMR, and toxin-induced changes in bilayer morphology were examined by freeze-fracture electron microscopy. Both NMR and EM showed the formation of an additional lipid phase in sphingomyelin-containing mixed lipid multilamellar suspensions with 0.4 mol % EqtII. The new toxin-induced phase consisted of small unilamellar vesicles 20-40 nm in diameter. Deuterium NMR showed that the new lipid phase contains both dimyristoylphosphatidycholine and sphingomyelin. Solid-state (31)P NMR showed an increase in spin-lattice and a decrease in spin-spin relaxation times in mixed-lipid model membranes in the presence of EqtII, consistent with an increase in the intensity of low frequency motions. The (2)H and (31)P spectral intensity distributions confirmed a change in lipid mobility and showed the creation of an isotropic lipid phase, which was identified as the small vesicle structures visible by electron microscopy in the EqtII-lipid suspensions. The toxin appears to enhance slow motions in the membrane lipids and destabilize the membrane. This effect was greatly enhanced in sphingomyelin-containing mixed lipid membranes compared with pure phosphatidylcholine bilayers, suggesting a preferential interaction between the toxin and bilayer sphingomyelin.
Figures

Similar articles
-
Neutron reflection study of the interaction of the eukaryotic pore-forming actinoporin equinatoxin II with lipid membranes reveals intermediate states in pore formation.Biochim Biophys Acta. 2016 Apr;1858(4):640-52. doi: 10.1016/j.bbamem.2015.12.019. Epub 2015 Dec 17. Biochim Biophys Acta. 2016. PMID: 26706098
-
Solid-state NMR study of membrane interactions of the pore-forming cytolysin, equinatoxin II.Biochim Biophys Acta. 2010 Feb;1798(2):244-51. doi: 10.1016/j.bbamem.2009.10.012. Epub 2009 Oct 24. Biochim Biophys Acta. 2010. PMID: 19857458
-
Solid-state NMR and simulation studies of equinatoxin II N-terminus interaction with lipid bilayers.Proteins. 2010 Mar;78(4):858-72. doi: 10.1002/prot.22612. Proteins. 2010. PMID: 19847922
-
Mechanism of action of equinatoxin II, a cytolysin from the sea anemone Actinia equina L. belonging to the family of actinoporins.Toxicology. 1994 Feb 28;87(1-3):205-27. doi: 10.1016/0300-483x(94)90252-6. Toxicology. 1994. PMID: 7512761 Review.
-
Sticholysins, two pore-forming toxins produced by the Caribbean Sea anemone Stichodactyla helianthus: their interaction with membranes.Toxicon. 2009 Dec 15;54(8):1135-47. doi: 10.1016/j.toxicon.2009.02.022. Epub 2009 Mar 4. Toxicon. 2009. PMID: 19268489 Review.
Cited by
-
A RNA-seq approach to identify putative toxins from acrorhagi in aggressive and non-aggressive Anthopleura elegantissima polyps.BMC Genomics. 2015 Mar 21;16(1):221. doi: 10.1186/s12864-015-1417-4. BMC Genomics. 2015. PMID: 25886045 Free PMC article.
-
Membrane Dynamics and Remodelling in Response to the Action of the Membrane-Damaging Pore-Forming Toxins.J Membr Biol. 2022 Jun;255(2-3):161-173. doi: 10.1007/s00232-022-00227-z. Epub 2022 Mar 19. J Membr Biol. 2022. PMID: 35305136 Review.
-
Insights on the interactions of synthetic amphipathic peptides with model membranes as revealed by 31P and 2H solid-state NMR and infrared spectroscopies.Biophys J. 2006 Jun 1;90(11):4071-84. doi: 10.1529/biophysj.105.077339. Epub 2006 Mar 13. Biophys J. 2006. PMID: 16533836 Free PMC article.
-
Determining the mode of action involved in the antimicrobial activity of synthetic peptides: a solid-state NMR and FTIR study.Biophys J. 2012 Oct 3;103(7):1470-9. doi: 10.1016/j.bpj.2012.08.055. Epub 2012 Oct 2. Biophys J. 2012. PMID: 23062339 Free PMC article.
-
Specific RNA binding to ordered phospholipid bilayers.Nucleic Acids Res. 2006 Apr 26;34(7):2128-36. doi: 10.1093/nar/gkl220. Print 2006. Nucleic Acids Res. 2006. PMID: 16641318 Free PMC article.
References
-
- Agirre, A., C. Flach, F. M. Goni, R. Mendelsohn, J. M. Valpuesta, F. J. Wu, and J. L. Nieva. 2000. Interactions of the HIV-1 fusion peptide with large unilamellar vesicles and monolayers. A cryo-TEM and spectroscopic study. Biochim. Biophys. Acta. 1467:153–164. - PubMed
-
- Anderluh, G., A. Barlic, Z. Podlesek, P. Macek, J. Pungercar, F. Gubensek, M. L. Zecchini, M. D. Serra, and G. Menestrina. 1999. Cysteine-scanning mutagenesis of an eukaryotic pore-forming toxin from sea anemone: topology in lipid membranes. Eur. J. Biochem. 263:128–136. - PubMed
-
- Anderluh, G., and P. Macek. 2002. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon. 40:111–124. - PubMed
-
- Anderluh, G., J. Pungercar, B. Strukelj, P. Macek, and F. Gubensek. 1996. Cloning, sequencing and expression of equinatoxin II. Biochem. Biophys. Res. Commun. 220:437–442. - PubMed
-
- Athanasiadis, A., G. Anderluh, P. Macek, and D. Turk. 2001. Crystal structure of the soluble form of Equinatoxin II, a pore-forming toxin from the sea anemone Actinia equina. Structure. 9:341–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

