Thyroid hormone-regulated enhancer blocking: cooperation of CTCF and thyroid hormone receptor
- PMID: 12660164
- PMCID: PMC152892
- DOI: 10.1093/emboj/cdg147
Thyroid hormone-regulated enhancer blocking: cooperation of CTCF and thyroid hormone receptor
Abstract
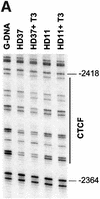
The highly conserved, ubiquitously expressed, zinc finger protein CTCF is involved in enhancer blocking, a mechanism crucial for shielding genes from illegitimate enhancer effects. Interestingly, CTCF-binding sites are often flanked by thyroid hormone response elements (TREs), as at the chicken lysozyme upstream silencer. Here we identify a similar composite site positioned upstream of the human c-myc gene. For both elements, we demonstrate that thyroid hormone abrogates enhancer blocking. Relief of enhancer blocking occurs even though CTCF remains bound to the lysozyme chromatin. Furthermore, chromatin immunoprecipitation analysis of the lysozyme upstream region revealed that histone H4 is acetylated at the CTCF-binding site. Loss of enhancer blocking by the addition of T3 led to increased histone acetylation, not only at the CTCF site, but also at the enhancer and the promoter. Thus, when TREs are adjacent to CTCF-binding sites, thyroid hormone can regulate enhancer blocking, thereby providing a new property for what was previously thought to be constitutive enhancer shielding by CTCF.
Figures






Similar articles
-
Role of CCAAT/enhancer-binding protein, histone acetylation, and coactivator recruitment in the regulation of malic enzyme transcription by thyroid hormone.Mol Cell Endocrinol. 2005 Dec 21;245(1-2):43-52. doi: 10.1016/j.mce.2005.10.002. Epub 2005 Nov 15. Mol Cell Endocrinol. 2005. PMID: 16293364
-
Modular insulators: genome wide search for composite CTCF/thyroid hormone receptor binding sites.PLoS One. 2010 Apr 9;5(4):e10119. doi: 10.1371/journal.pone.0010119. PLoS One. 2010. PMID: 20404925 Free PMC article.
-
CTCF-dependent chromatin insulator is linked to epigenetic remodeling.Mol Cell. 2006 Sep 1;23(5):733-42. doi: 10.1016/j.molcel.2006.08.008. Mol Cell. 2006. PMID: 16949368
-
CTCF function is modulated by neighboring DNA binding factors.Biochem Cell Biol. 2011 Oct;89(5):459-68. doi: 10.1139/o11-033. Epub 2011 Sep 7. Biochem Cell Biol. 2011. PMID: 21895576 Review.
-
The thyroid hormone receptor and the insulator protein CTCF: two different factors with overlapping functions.J Steroid Biochem Mol Biol. 2002 Dec;83(1-5):49-57. doi: 10.1016/s0960-0760(02)00256-x. J Steroid Biochem Mol Biol. 2002. PMID: 12650701 Review.
Cited by
-
Chromatin fine structure of the c-MYC insulator element/DNase I-hypersensitive site I is not preserved during mitosis.Proc Natl Acad Sci U S A. 2007 Oct 2;104(40):15741-6. doi: 10.1073/pnas.0702363104. Epub 2007 Sep 21. Proc Natl Acad Sci U S A. 2007. PMID: 17890321 Free PMC article.
-
CTCF is conserved from Drosophila to humans and confers enhancer blocking of the Fab-8 insulator.EMBO Rep. 2005 Feb;6(2):165-70. doi: 10.1038/sj.embor.7400334. EMBO Rep. 2005. PMID: 15678159 Free PMC article.
-
Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):E766-75. doi: 10.1073/pnas.1210626110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382204 Free PMC article.
-
Developmental activation of the lysozyme gene in chicken macrophage cells is linked to core histone acetylation at its enhancer elements.Nucleic Acids Res. 2006;34(14):4025-35. doi: 10.1093/nar/gkl543. Epub 2006 Aug 16. Nucleic Acids Res. 2006. PMID: 16914441 Free PMC article.
-
Epigenetic boundaries of tumour suppressor gene promoters: the CTCF connection and its role in carcinogenesis.J Cell Mol Med. 2006 Jul-Sep;10(3):554-68. doi: 10.1111/j.1582-4934.2006.tb00420.x. J Cell Mol Med. 2006. PMID: 16989720 Free PMC article. Review.
References
-
- Aranda A. and Pascual,A. (2001) Nuclear hormone receptors and gene expression. Physiol. Rev., 81, 1269–1304. - PubMed
-
- Awad T.A. et al. (1999) Negative transcriptional regulation mediated by thyroid hormone response element 144 requires binding of the multivalent factor CTCF to a novel target DNA sequence. J. Biol. Chem., 274, 27092–27098. - PubMed
-
- Baniahmad A., Steiner,C., Koehne,A.C. and Renkawitz,R. (1990) Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell, 61, 505–514. - PubMed
-
- Belandia B., Latasa,M.J., Villa,A. and Pascual,A. (1998) Thyroid hormone negatively regulates the transcriptional activity of the β-amyloid precursor protein gene. J. Biol. Chem., 273, 30366–30371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

