Links between signal transduction, transcription and adhesion in epithelial bud development
- PMID: 12646922
- PMCID: PMC2424170
- DOI: 10.1038/nature01458
Links between signal transduction, transcription and adhesion in epithelial bud development
Erratum in
- Nature. 2003 Aug 21;424(6951):974
Abstract
The morphogenesis of organs as diverse as lungs, teeth and hair follicles is initiated by a downgrowth from a layer of epithelial stem cells. During follicular morphogenesis, stem cells form this bud structure by changing their polarity and cell-cell contacts. Here we show that this process is achieved through simultaneous receipt of two external signals: a Wnt protein to stabilize beta-catenin, and a bone morphogenetic protein (BMP) inhibitor to produce Lef1. Beta-catenin then binds to, and activates, Lef1 transcription complexes that appear to act uncharacteristically by downregulating the gene encoding E-cadherin, an important component of polarity and intercellular adhesion. When either signal is missing, functional Lef1 complexes are not made, and E-cadherin downregulation and follicle morphogenesis are impaired. In Drosophila, E-cadherin can influence the plane of cell division and cytoskeletal dynamics. Consistent with this notion, we show that forced elevation of E-cadherin levels block invagination and follicle production. Our findings reveal an intricate molecular programme that links two extracellular signalling pathways to the formation of a nuclear transcription factor that acts on target genes to remodel cellular junctions and permit follicle formation.
Figures
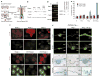

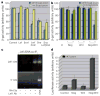
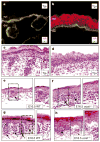
Comment in
-
Developmental biology: A hairy situation.Nature. 2003 Mar 20;422(6929):272-3. doi: 10.1038/422272a. Nature. 2003. PMID: 12646907 No abstract available.
Similar articles
-
Developmental biology: A hairy situation.Nature. 2003 Mar 20;422(6929):272-3. doi: 10.1038/422272a. Nature. 2003. PMID: 12646907 No abstract available.
-
Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA.J Cell Biol. 2003 Nov 10;163(3):609-23. doi: 10.1083/jcb.200309042. J Cell Biol. 2003. PMID: 14610062 Free PMC article.
-
Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation.Development. 1999 Oct;126(20):4557-68. doi: 10.1242/dev.126.20.4557. Development. 1999. PMID: 10498690
-
Convergence of Wnt, beta-catenin, and cadherin pathways.Science. 2004 Mar 5;303(5663):1483-7. doi: 10.1126/science.1094291. Science. 2004. PMID: 15001769 Free PMC article. Review.
-
Cell adhesion system and human cancer morphogenesis.Cancer Sci. 2003 Jul;94(7):575-81. doi: 10.1111/j.1349-7006.2003.tb01485.x. Cancer Sci. 2003. PMID: 12841864 Free PMC article. Review.
Cited by
-
Tetraspanin18 is a FoxD3-responsive antagonist of cranial neural crest epithelial-to-mesenchymal transition that maintains cadherin-6B protein.J Cell Sci. 2013 Mar 15;126(Pt 6):1464-76. doi: 10.1242/jcs.120915. Epub 2013 Feb 15. J Cell Sci. 2013. PMID: 23418345 Free PMC article.
-
Transcriptome and proteome characterization of surface ectoderm cells differentiated from human iPSCs.Sci Rep. 2016 Aug 23;6:32007. doi: 10.1038/srep32007. Sci Rep. 2016. PMID: 27550649 Free PMC article.
-
The way Wnt works: components and mechanism.Growth Factors. 2013 Feb;31(1):1-31. doi: 10.3109/08977194.2012.752737. Epub 2012 Dec 21. Growth Factors. 2013. PMID: 23256519 Free PMC article. Review.
-
Long non-coding RNA SNHG4 enhances RNF14 mRNA stability to promote the progression of colorectal cancer by recruiting TAF15 protein.Apoptosis. 2023 Apr;28(3-4):414-431. doi: 10.1007/s10495-022-01781-6. Epub 2022 Dec 9. Apoptosis. 2023. PMID: 36482019
-
Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation.Dev Biol. 2004 Feb 1;266(1):109-22. doi: 10.1016/j.ydbio.2003.10.010. Dev Biol. 2004. PMID: 14729482 Free PMC article.
References
-
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. - PubMed
-
- Hogan BL. Morphogenesis. Cell. 1999;96:225–233. - PubMed
-
- Jan Y-N, Jan L-Y. Asymmetric cell division in the Drosophila nervous system. Nature Rev Neurosci. 2001;2:772–779. - PubMed
-
- van Genderen C, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. - PubMed
-
- Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:700–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

