Involvement of poly(ADP-Ribose) polymerase 1 and poly(ADP-Ribosyl)ation in regulation of centrosome function
- PMID: 12640128
- PMCID: PMC150716
- DOI: 10.1128/MCB.23.7.2451-2462.2003
Involvement of poly(ADP-Ribose) polymerase 1 and poly(ADP-Ribosyl)ation in regulation of centrosome function
Abstract
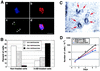
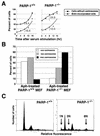
The regulatory mechanism of centrosome function is crucial to the accurate transmission of chromosomes to the daughter cells in mitosis. Recent findings on the posttranslational modifications of many centrosomal proteins led us to speculate that these modifications might be involved in centrosome behavior. Poly(ADP-ribose) polymerase 1 (PARP-1) catalyzes poly(ADP-ribosyl)ation to various proteins. We show here that PARP-1 localizes to centrosomes and catalyzes poly(ADP-ribosyl)ation of centrosomal proteins. Moreover, centrosome hyperamplification is frequently observed with PARP inhibitor, as well as in PARP-1-null cells. Thus, it is possible that chromosomal instability known in PARP-1-null cells can be attributed to the centrosomal dysfunction. P53 tumor suppressor protein has been also shown to be localized at centrosomes and to be involved in the regulation of centrosome duplication and monitoring of the chromosomal stability. We found that centrosomal p53 is poly(ADP-ribosyl)ated in vivo and centrosomal PARP-1 directly catalyzes poly(ADP-ribosyl)ation of p53 in vitro. These results indicate that PARP-1 and PARP-1-mediated poly(ADP-ribosyl)ation of centrosomal proteins are involved in the regulation of centrosome function.
Figures





Similar articles
-
Haploinsufficiency of poly(ADP-ribose) polymerase-1-mediated poly(ADP-ribosyl)ation for centrosome duplication.Biochem Biophys Res Commun. 2007 Aug 3;359(3):426-30. doi: 10.1016/j.bbrc.2007.05.108. Epub 2007 May 25. Biochem Biophys Res Commun. 2007. PMID: 17553458
-
Transient poly(ADP-ribosyl)ation of nuclear proteins and role of poly(ADP-ribose) polymerase in the early stages of apoptosis.J Biol Chem. 1998 May 29;273(22):13703-12. doi: 10.1074/jbc.273.22.13703. J Biol Chem. 1998. PMID: 9593711
-
Poly(ADP-ribose) polymerase inhibitors activate the p53 signaling pathway in neural stem/progenitor cells.BMC Neurosci. 2017 Jan 17;18(1):14. doi: 10.1186/s12868-016-0333-0. BMC Neurosci. 2017. PMID: 28095779 Free PMC article.
-
Poly(ADP-ribosyl)ation, PARP, and aging.Sci Aging Knowledge Environ. 2004 Dec 8;2004(49):re9. doi: 10.1126/sageke.2004.49.re9. Sci Aging Knowledge Environ. 2004. PMID: 15590998 Review.
-
Poly(ADP-ribosyl)ation, genomic instability, and longevity.Ann N Y Acad Sci. 2000 Jun;908:126-32. doi: 10.1111/j.1749-6632.2000.tb06641.x. Ann N Y Acad Sci. 2000. PMID: 10911953 Review.
Cited by
-
Enhanced Cytotoxicity on Cancer Cells by Combinational Treatment of PARP Inhibitor and 5-Azadeoxycytidine Accompanying Distinct Transcriptional Profiles.Cancers (Basel). 2022 Aug 28;14(17):4171. doi: 10.3390/cancers14174171. Cancers (Basel). 2022. PMID: 36077707 Free PMC article.
-
Biological processes and signal transduction pathways regulated by the protein methyltransferase SETD7 and their significance in cancer.Signal Transduct Target Ther. 2018 Jul 13;3:19. doi: 10.1038/s41392-018-0017-6. eCollection 2018. Signal Transduct Target Ther. 2018. PMID: 30013796 Free PMC article.
-
Beyond DNA Repair: Additional Functions of PARP-1 in Cancer.Front Oncol. 2013 Nov 27;3:290. doi: 10.3389/fonc.2013.00290. Front Oncol. 2013. PMID: 24350055 Free PMC article. Review.
-
Linking DNA repair and cell cycle progression through serine ADP-ribosylation of histones.Nat Commun. 2022 Jan 13;13(1):185. doi: 10.1038/s41467-021-27867-4. Nat Commun. 2022. PMID: 35027540 Free PMC article.
-
The roles of PARP1 in gene control and cell differentiation.Curr Opin Genet Dev. 2010 Oct;20(5):512-8. doi: 10.1016/j.gde.2010.06.001. Epub 2010 Jun 28. Curr Opin Genet Dev. 2010. PMID: 20591646 Free PMC article. Review.
References
-
- Adolph, K. W., and M. K. Song. 1985. Decrease in ADP-ribosylation of HeLa non-histone proteins from interphase to metaphase. Biochemistry 24:345-352. - PubMed
-
- Amstad, P. A., G. Krupitza, and P. A. Cerutti. 1992. Mechanism of c-fos induction by active oxygen. Cancer Res. 52:3952-3960. - PubMed
-
- Blair Zajdel, M. E., and G. E. Blair. 1988. The intracellular distribution of the transformation-associated protein p53 in adenovirus-transformed rodent cells. Oncogene 2:579-584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
