GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif
- PMID: 12631717
- PMCID: PMC151573
- DOI: 10.1091/mbc.e02-06-0315
GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif
Abstract
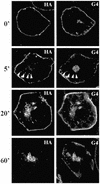
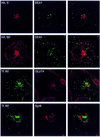
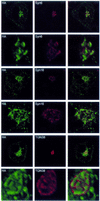
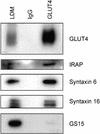
Insulin stimulates glucose transport in fat and muscle cells by triggering exocytosis of the glucose transporter GLUT4. To define the intracellular trafficking of GLUT4, we have studied the internalization of an epitope-tagged version of GLUT4 from the cell surface. GLUT4 rapidly traversed the endosomal system en route to a perinuclear location. This perinuclear GLUT4 compartment did not colocalize with endosomal markers (endosomal antigen 1 protein, transferrin) or TGN38, but showed significant overlap with the TGN target (t)-soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) Syntaxins 6 and 16. These results were confirmed by vesicle immunoisolation. Consistent with a role for Syntaxins 6 and 16 in GLUT4 trafficking we found that their expression was up-regulated significantly during adipocyte differentiation and insulin stimulated their movement to the cell surface. GLUT4 trafficking between endosomes and trans-Golgi network was regulated via an acidic targeting motif in the carboxy terminus of GLUT4, because a mutant lacking this motif was retained in endosomes. We conclude that GLUT4 is rapidly transported from the cell surface to a subdomain of the trans-Golgi network that is enriched in the t-SNAREs Syntaxins 6 and 16 and that an acidic targeting motif in the C-terminal tail of GLUT4 plays an important role in this process.
Figures

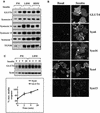

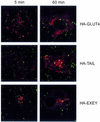

Similar articles
-
Adipsin and the glucose transporter GLUT4 traffic to the cell surface via independent pathways in adipocytes.Traffic. 2000 Feb;1(2):141-51. doi: 10.1034/j.1600-0854.2000.010206.x. Traffic. 2000. PMID: 11208094
-
Analysis of the co-localization of the insulin-responsive glucose transporter (GLUT4) and the trans Golgi network marker TGN38 within 3T3-L1 adipocytes.Biochem J. 1994 Jun 15;300 ( Pt 3)(Pt 3):743-9. doi: 10.1042/bj3000743. Biochem J. 1994. PMID: 8010955 Free PMC article.
-
Insulin recruits GLUT4 from specialized VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes.Mol Biol Cell. 2000 Dec;11(12):4079-91. doi: 10.1091/mbc.11.12.4079. Mol Biol Cell. 2000. PMID: 11102509 Free PMC article.
-
Targeting motifs in GLUT4 (review).Mol Membr Biol. 2001 Oct-Dec;18(4):257-64. doi: 10.1080/09687680110090780. Mol Membr Biol. 2001. PMID: 11780754 Review.
-
Role of SNARE's in the GLUT4 translocation response to insulin in adipose cells and muscle.J Basic Clin Physiol Pharmacol. 1998;9(2-4):153-65. doi: 10.1515/jbcpp.1998.9.2-4.153. J Basic Clin Physiol Pharmacol. 1998. PMID: 10212832 Review.
Cited by
-
COG complexes form spatial landmarks for distinct SNARE complexes.Nat Commun. 2013;4:1553. doi: 10.1038/ncomms2535. Nat Commun. 2013. PMID: 23462996 Free PMC article.
-
An ACAP1-containing clathrin coat complex for endocytic recycling.J Cell Biol. 2007 Jul 30;178(3):453-64. doi: 10.1083/jcb.200608033. J Cell Biol. 2007. PMID: 17664335 Free PMC article.
-
The first luminal loop confers insulin responsiveness to glucose transporter 4.Mol Biol Cell. 2012 Mar;23(5):910-7. doi: 10.1091/mbc.E11-10-0839. Epub 2012 Jan 19. Mol Biol Cell. 2012. PMID: 22262463 Free PMC article.
-
Syntaxin 16's Newly Deciphered Roles in Autophagy.Cells. 2019 Dec 17;8(12):1655. doi: 10.3390/cells8121655. Cells. 2019. PMID: 31861136 Free PMC article. Review.
-
Cluster analysis of insulin action in adipocytes reveals a key role for Akt at the plasma membrane.J Biol Chem. 2010 Jan 22;285(4):2245-57. doi: 10.1074/jbc.M109.060236. Epub 2009 Nov 6. J Biol Chem. 2010. PMID: 19897488 Free PMC article.
References
-
- Bennett G, O'Shaughnessy D. The site of incorporation of sialic acid residues into glycoproteins and the subsequent fates of these molecules in various rat and mouse cell types as shown by radioautography after injection of [3H]N-acetylmannosamine. I. Observations in hepatocytes. J Cell Biol. 1981;88:1–15. - PMC - PubMed
-
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter glut4. Nat Rev Mol Cell Biol. 2002;3:267–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

