Sensory neuron subtypes have unique substratum preference and receptor expression before target innervation
- PMID: 12629182
- PMCID: PMC6741987
- DOI: 10.1523/JNEUROSCI.23-05-01781.2003
Sensory neuron subtypes have unique substratum preference and receptor expression before target innervation
Abstract
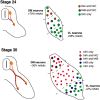
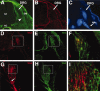
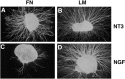
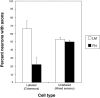
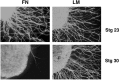
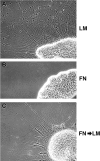
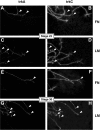
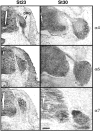
The factors controlling the specification and subsequent differentiation of sensory neurons are poorly understood. Data from embryological manipulations suggest that either sensory neuron fates are specified by the targets they encounter or sensory neurons are considerably more "plastic" with respect to specification than are neurons of the CNS. The prevailing view that sensory neurons are specified late in development is not consistent, however, with the directed outgrowth of sensory neurons to their targets and the characteristic spatial distribution of sensory neuron fates within the peripheral ganglia. To address when in development different classes of sensory neurons can first be distinguished, we investigated the interactions of early dorsal root ganglia neurons with the extracellular matrix before neurite outgrowth to targets. We found that subclasses of sensory neurons in early dorsal root ganglia show different patterns of neurite outgrowth and integrin expression that are predictive of their fates. In the absence of neurotrophins, presumptive proprioceptive neurons extend neurites robustly on both laminin and fibronectin, whereas presumptive cutaneous neurons show a strong preference for laminin. Cutaneous afferents that have innervated targets show a similar strong preference for laminin and show higher levels of integrin alpha7beta1 than do proprioceptive neurons. Finally, presumptive proprioceptive neurons express fibronectin receptors, integrin alpha3beta1, alpha4beta1, and alpha5beta1, at higher levels than do presumptive cutaneous neurons. Our results indicate that subtypes of sensory neurons have unique patterns of neurite outgrowth and receptor expression before target innervation.
Figures

Similar articles
-
Neurotrophins and extracellular matrix molecules modulate sensory axon outgrowth.Int J Dev Neurosci. 2004 Apr;22(2):113-7. doi: 10.1016/j.ijdevneu.2003.12.002. Int J Dev Neurosci. 2004. PMID: 15036386
-
Substratum preferences of motor and sensory neurons in postnatal and adult rats.Eur J Neurosci. 2016 Feb;43(3):431-42. doi: 10.1111/ejn.13057. Epub 2015 Sep 30. Eur J Neurosci. 2016. PMID: 26332537
-
Expression of beta 1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms.J Neurosci. 1993 Nov;13(11):4880-8. doi: 10.1523/JNEUROSCI.13-11-04880.1993. J Neurosci. 1993. PMID: 7693896 Free PMC article.
-
Local and target-derived actions of neurotrophins during peripheral nervous system development.Cell Mol Life Sci. 2001 Jul;58(8):1036-44. doi: 10.1007/PL00000918. Cell Mol Life Sci. 2001. PMID: 11529496 Free PMC article. Review.
-
Generation of somatic sensory neuron diversity and implications on sensory coding.Curr Opin Neurobiol. 2011 Feb;21(1):52-60. doi: 10.1016/j.conb.2010.09.003. Epub 2010 Oct 1. Curr Opin Neurobiol. 2011. PMID: 20888752 Free PMC article. Review.
Cited by
-
CSPGs inhibit axon branching by impairing mitochondria-dependent regulation of actin dynamics and axonal translation.Dev Neurobiol. 2017 Apr;77(4):454-473. doi: 10.1002/dneu.22420. Epub 2016 Aug 2. Dev Neurobiol. 2017. PMID: 27429169 Free PMC article.
-
Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions.Dev Neurobiol. 2011 Nov;71(11):901-23. doi: 10.1002/dneu.20931. Dev Neurobiol. 2011. PMID: 21714101 Free PMC article. Review.
-
Communicating pain: emerging axonal signaling in peripheral neuropathic pain.Front Neuroanat. 2024 Jul 9;18:1398400. doi: 10.3389/fnana.2024.1398400. eCollection 2024. Front Neuroanat. 2024. PMID: 39045347 Free PMC article. Review.
-
Retinoic acid influences anteroposterior positioning of epidermal sensory neurons and their gene expression in a developing chordate (amphioxus).Proc Natl Acad Sci U S A. 2004 Jul 13;101(28):10320-5. doi: 10.1073/pnas.0403216101. Epub 2004 Jun 29. Proc Natl Acad Sci U S A. 2004. PMID: 15226493 Free PMC article.
-
Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia.J Neurosci. 2010 Sep 8;30(36):12185-97. doi: 10.1523/JNEUROSCI.1740-10.2010. J Neurosci. 2010. PMID: 20826681 Free PMC article.
References
-
- Adams DH, Scott SA. Response of “naive” cutaneous and muscle afferents to potential targets in vitro. Dev Biol. 1998;203:210–220. - PubMed
-
- Anderson DJ. Lineages and transcription factors in the specification of vertebrate primary sensory neurons. Curr Opin Neurobiol. 1999;9:517–524. - PubMed
-
- Aumailley M, Rousselle P. Laminins of the dermo-epidermal junction. Matrix Biol. 1999;18:19–28. - PubMed
-
- Backstrom A, Soderstrom S, Kylberg A, Ebendal T. Molecular cloning of the chicken trkA and its expression in early peripheral ganglia. J Neurosci Res. 1996;46:67–81. - PubMed
-
- Baker CV, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

