Widespread defects in the primary olfactory pathway caused by loss of Mash1 function
- PMID: 12629181
- PMCID: PMC6741991
- DOI: 10.1523/JNEUROSCI.23-05-01769.2003
Widespread defects in the primary olfactory pathway caused by loss of Mash1 function
Abstract
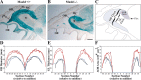
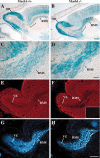
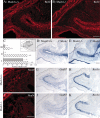
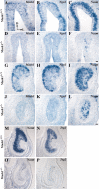
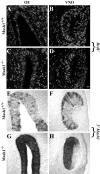
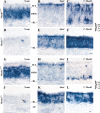
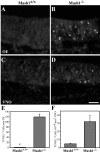
MASH1, a basic helix-loop-helix transcription factor, is widely expressed by neuronal progenitors in the CNS and PNS, suggesting that it plays a role in the development of many neural regions. However, in mice lacking a functional Mash1 gene, major alterations have been reported in only a few neuronal populations; among these is a generalized loss of olfactory receptor neurons of the olfactory epithelium. Here, we use a transgenic reporter mouse line, in which the cell bodies and growing axons of subsets of central and peripheral neurons are marked by expression of a tau-lacZ reporter gene (the Tattler-4 allele), to look both more broadly and deeply at defects in the nervous system of Mash1-/- mice. In addition to the expected lack of olfactory receptor neurons in the main olfactory epithelium, developing Mash1-/-;Tattler-4+/- mice exhibited reductions in neuronal cell number in the vomeronasal organ and in the olfactory bulb; the morphology of the rostral migratory stream, which gives rise to olfactory bulb interneurons, was also abnormal. Further examination of cell proliferation, cell death, and cell type-specific markers in Mash1-/- animals uncovered parallels between the main olfactory epithelium and the vomeronasal organ in the regulation of sensory neuron development. Interestingly, this analysis also revealed that, in the olfactory epithelium of Mash1-/- animals, there is an overproduction of proliferating cells that co-express markers of both neuronal progenitors and supporting cells. This finding suggests that olfactory receptor neurons and olfactory epithelium supporting cells may share a common progenitor, and that expression of Mash1 may be an important factor in determining whether these progenitors ultimately generate neurons or glia.
Figures


Similar articles
-
Lineage-specific regulation of the neural differentiation gene MASH1.Dev Biol. 1996 Dec 15;180(2):605-17. doi: 10.1006/dbio.1996.0332. Dev Biol. 1996. PMID: 8954731
-
Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors.Development. 1997 Apr;124(8):1611-21. doi: 10.1242/dev.124.8.1611. Development. 1997. PMID: 9108377
-
Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis.Eur J Neurosci. 2009 Jan;29(2):232-43. doi: 10.1111/j.1460-9568.2008.06595.x. Eur J Neurosci. 2009. PMID: 19200230
-
bHLH transcription factors and mammalian neuronal differentiation.Int J Biochem Cell Biol. 1997 Dec;29(12):1389-99. doi: 10.1016/s1357-2725(97)89968-2. Int J Biochem Cell Biol. 1997. PMID: 9570134 Review.
-
Cell migration in the developing rodent olfactory system.Cell Mol Life Sci. 2016 Jul;73(13):2467-90. doi: 10.1007/s00018-016-2172-7. Epub 2016 Mar 18. Cell Mol Life Sci. 2016. PMID: 26994098 Free PMC article. Review.
Cited by
-
Retinoic acid regulates olfactory progenitor cell fate and differentiation.Neural Dev. 2013 Jul 5;8:13. doi: 10.1186/1749-8104-8-13. Neural Dev. 2013. PMID: 23829703 Free PMC article.
-
Visualizing Cell Cycle Phase Organization and Control During Neural Lineage Elaboration.Cells. 2020 Sep 17;9(9):2112. doi: 10.3390/cells9092112. Cells. 2020. PMID: 32957483 Free PMC article.
-
PBK/TOPK, a proliferating neural progenitor-specific mitogen-activated protein kinase kinase.J Neurosci. 2005 Nov 16;25(46):10773-85. doi: 10.1523/JNEUROSCI.3207-05.2005. J Neurosci. 2005. PMID: 16291951 Free PMC article.
-
The prokineticins: a novel pair of regulatory peptides.Mol Interv. 2006 Dec;6(6):330-8. doi: 10.1124/mi.6.6.6. Mol Interv. 2006. PMID: 17200460 Free PMC article. Review.
-
Fezf1 and Fezf2 are required for olfactory development and sensory neuron identity.J Comp Neurol. 2011 Jul 1;519(10):1829-46. doi: 10.1002/cne.22596. J Comp Neurol. 2011. PMID: 21452247 Free PMC article.
References
-
- Alvarez-Buylla A. Mechanism of migration of olfactory bulb interneurons. Semin Cell Dev Biol. 1997;8:207–213. - PubMed
-
- Angevine JB., Jr Time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J Comp Neurol. 1970;139:129–187. - PubMed
-
- Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, Gershon MD. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Development. 1996;122:309–320. - PubMed
-
- Brunet JF, Ghysen A. Deconstructing cell determination: proneural genes and neuronal identity. BioEssays. 1999;21:313–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

