High levels of intracellular cysteine promote oxidative DNA damage by driving the fenton reaction
- PMID: 12618458
- PMCID: PMC150142
- DOI: 10.1128/JB.185.6.1942-1950.2003
High levels of intracellular cysteine promote oxidative DNA damage by driving the fenton reaction
Abstract
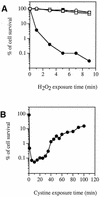
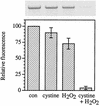
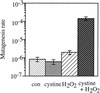
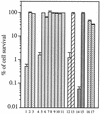
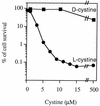
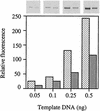
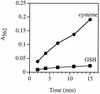
Escherichia coli is generally resistant to H(2)O(2), with >75% of cells surviving a 3-min challenge with 2.5 mM H(2)O(2). However, when cells were cultured with poor sulfur sources and then exposed to cystine, they transiently exhibited a greatly increased susceptibility to H(2)O(2), with <1% surviving the challenge. Cell death was due to an unusually rapid rate of DNA damage, as indicated by their filamentation, a high rate of mutation among the survivors, and DNA lesions by a direct assay. Cell-permeable iron chelators eliminated sensitivity, indicating that intracellular free iron mediated the conversion of H(2)O(2) into a hydroxyl radical, the direct effector of DNA damage. The cystine treatment caused a temporary loss of cysteine homeostasis, with intracellular pools increasing about eightfold. In vitro analysis demonstrated that cysteine reduces ferric iron with exceptional speed. This action permits free iron to redox cycle rapidly in the presence of H(2)O(2), thereby augmenting the rate at which hydroxyl radicals are formed. During routine growth, cells maintain small cysteine pools, and cysteine is not a major contributor to DNA damage. Thus, the homeostatic control of cysteine levels is important in conferring resistance to oxidants. More generally, this study provides a new example of a situation in which the vulnerability of cells to oxidative DNA damage is strongly affected by their physiological state.
Figures


Similar articles
-
Reduced flavins promote oxidative DNA damage in non-respiring Escherichia coli by delivering electrons to intracellular free iron.J Biol Chem. 2002 Sep 13;277(37):34055-66. doi: 10.1074/jbc.M203977200. Epub 2002 Jun 21. J Biol Chem. 2002. PMID: 12080063
-
The YaaA protein of the Escherichia coli OxyR regulon lessens hydrogen peroxide toxicity by diminishing the amount of intracellular unincorporated iron.J Bacteriol. 2011 May;193(9):2186-96. doi: 10.1128/JB.00001-11. Epub 2011 Mar 4. J Bacteriol. 2011. PMID: 21378183 Free PMC article.
-
A mechanism by which nitric oxide accelerates the rate of oxidative DNA damage in Escherichia coli.Mol Microbiol. 2003 Jul;49(1):11-22. doi: 10.1046/j.1365-2958.2003.03530.x. Mol Microbiol. 2003. PMID: 12823807
-
DNA damage and oxygen radical toxicity.Science. 1988 Jun 3;240(4857):1302-9. doi: 10.1126/science.3287616. Science. 1988. PMID: 3287616 Review.
-
Avoiding high-valent iron intermediates: superoxide reductase and rubrerythrin.J Inorg Biochem. 2006 Apr;100(4):679-93. doi: 10.1016/j.jinorgbio.2005.12.017. Epub 2006 Feb 28. J Inorg Biochem. 2006. PMID: 16504301 Review.
Cited by
-
Cyanide enhances hydrogen peroxide toxicity by recruiting endogenous iron to trigger catastrophic chromosomal fragmentation.Mol Microbiol. 2015 Apr;96(2):349-67. doi: 10.1111/mmi.12938. Epub 2015 Feb 18. Mol Microbiol. 2015. PMID: 25598241 Free PMC article.
-
Fluoroquinolone heteroresistance, antimicrobial tolerance, and lethality enhancement.Front Cell Infect Microbiol. 2022 Sep 29;12:938032. doi: 10.3389/fcimb.2022.938032. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36250047 Free PMC article. Review.
-
Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and independent transcriptional networks.PLoS One. 2007 Nov 14;2(11):e1186. doi: 10.1371/journal.pone.0001186. PLoS One. 2007. PMID: 18000553 Free PMC article.
-
Bacillithiol, a new player in bacterial redox homeostasis.Antioxid Redox Signal. 2011 Jul 1;15(1):123-33. doi: 10.1089/ars.2010.3562. Epub 2010 Dec 17. Antioxid Redox Signal. 2011. PMID: 20712413 Free PMC article. Review.
-
Synthesis versus degradation: directions of amino acid metabolism during Arabidopsis abiotic stress response.Plant Mol Biol. 2018 Sep;98(1-2):121-135. doi: 10.1007/s11103-018-0767-0. Epub 2018 Aug 24. Plant Mol Biol. 2018. PMID: 30143990
References
-
- Alikhanian, S. I., T. S. Iljina, E. S. Kaliaeva, S. V. Kameneva, and V. V. Sukodolec. 1965. Mutants of Escherichia coli K12 lacking thymine. Nature 206:848-849. - PubMed
-
- Anderson, M. E. 1985. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 113:548-555. - PubMed
-
- Bachmann, B. J. 1987. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 1190-1219. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

