IkappaBalpha and p65 regulate the cytoplasmic shuttling of nuclear corepressors: cross-talk between Notch and NFkappaB pathways
- PMID: 12589049
- PMCID: PMC149987
- DOI: 10.1091/mbc.e02-07-0404
IkappaBalpha and p65 regulate the cytoplasmic shuttling of nuclear corepressors: cross-talk between Notch and NFkappaB pathways
Abstract
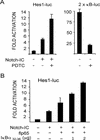
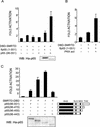
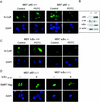
Notch and NFkappaB pathways are key regulators of numerous cellular events such as proliferation, differentiation, or apoptosis. In both pathways, association of effector proteins with nuclear corepressors is responsible for their negative regulation. We have previously described that expression of a p65-NFkappaB mutant that lacks the transactivation domain (p65DeltaTA) induces cytoplasmic translocation of N-CoR leading to a positive regulation of different promoters. Now, we show that cytoplasmic sequestration of p65 by IkappaBalpha is sufficient to both translocate nuclear corepressors SMRT/N-CoR to the cytoplasm and upregulate transcription of Notch-dependent genes. Moreover, p65 and IkappaBalpha are able to directly bind SMRT, and this interaction can be inhibited in a dose-dependent manner by the CREB binding protein (CBP) coactivator and after TNF-alpha treatment, suggesting that p65 acetylation is modulating this interaction. In agreement with this, TNF-alpha treatment results in downregulation of the Hes1 gene. Finally, we present evidence on how this mechanism may influence cell differentiation in the 32D myeloid progenitor system.
Figures

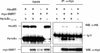

Similar articles
-
Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis.J Biol Chem. 2004 Apr 9;279(15):15096-104. doi: 10.1074/jbc.M311192200. Epub 2004 Jan 7. J Biol Chem. 2004. PMID: 14711835
-
p65-NFkappaB synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR.J Cell Sci. 2002 Mar 15;115(Pt 6):1295-303. doi: 10.1242/jcs.115.6.1295. J Cell Sci. 2002. PMID: 11884528
-
Tyrosine kinase p56lck regulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through tyrosine phosphorylation of IkappaBalpha following hypoxia/reoxygenation.J Biol Chem. 2003 Dec 26;278(52):52598-612. doi: 10.1074/jbc.M308941200. Epub 2003 Oct 8. J Biol Chem. 2003. PMID: 14534291
-
Osteopontin induces nuclear factor kappa B-mediated promatrix metalloproteinase-2 activation through I kappa B alpha /IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways.J Biol Chem. 2003 Apr 18;278(16):14487-97. doi: 10.1074/jbc.M207309200. Epub 2002 Dec 7. J Biol Chem. 2003. PMID: 12473670
-
Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation.Biochem Pharmacol. 2004 Sep 15;68(6):1221-9. doi: 10.1016/j.bcp.2004.05.039. Biochem Pharmacol. 2004. PMID: 15313420 Review.
Cited by
-
p65 Negatively regulates transcription of the cyclin E gene.J Biol Chem. 2010 Jun 4;285(23):17453-64. doi: 10.1074/jbc.M109.058974. Epub 2010 Apr 11. J Biol Chem. 2010. PMID: 20385564 Free PMC article.
-
Notch1 augments NF-kappaB activity by facilitating its nuclear retention.EMBO J. 2006 Jan 11;25(1):129-38. doi: 10.1038/sj.emboj.7600902. Epub 2005 Dec 1. EMBO J. 2006. PMID: 16319921 Free PMC article.
-
Transducin β-like protein 1 recruits nuclear factor κB to the target gene promoter for transcriptional activation.Mol Cell Biol. 2011 Mar;31(5):924-34. doi: 10.1128/MCB.00576-10. Epub 2010 Dec 28. Mol Cell Biol. 2011. PMID: 21189284 Free PMC article.
-
Inhibition of specific NF-κB activity contributes to the tumor suppressor function of 14-3-3σ in breast cancer.PLoS One. 2012;7(5):e38347. doi: 10.1371/journal.pone.0038347. Epub 2012 May 31. PLoS One. 2012. PMID: 22675457 Free PMC article.
-
TLR8: an innate immune receptor in brain, neurons and axons.Cell Cycle. 2007 Dec 1;6(23):2859-68. doi: 10.4161/cc.6.23.5018. Epub 2007 Sep 4. Cell Cycle. 2007. PMID: 18000403 Free PMC article. Review.
References
-
- Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-B and -amyloid precursor protein. Cell. 2002;110:55–67. - PubMed
-
- Bailey P, Downes M, Lau P, Harris J, Chen SL, Hamamori Y, Sartorelli V, Muscat GE. The nuclear receptor corepressor N-CoR regulates differentiation: N-CoR directly interacts with MyoD. Mol Endocrinol. 1999;13:1155–1168. - PubMed
-
- Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

