Wnts and TGF beta in synaptogenesis: old friends signalling at new places
- PMID: 12563282
- PMCID: PMC3503525
- DOI: 10.1038/nrn1036
Wnts and TGF beta in synaptogenesis: old friends signalling at new places
Abstract
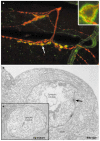
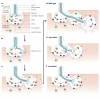
The formation of mature synaptic connections involves the targeted transport and aggregation of synaptic vesicles, the gathering of presynaptic release sites and the clustering of postsynaptic neurotransmitter receptors and ion channels. Positional cues are required to orient the cytoskeleton in the direction of neuronal outgrowth, and also to direct the juxtaposition of synaptic protein complexes at the pre- and postsynaptic membranes. Both anterograde and retrograde factors are thought to contribute positional information during synaptic differentiation, and recent studies in vertebrates and invertebrates have begun to uncover a new role in this process for proteins that are essential for pattern formation in the early embryo.
Figures

Similar articles
-
Synaptogenesis: Wnt and TGF-beta take centre stage.Curr Biol. 2003 Jan 21;13(2):R60-2. doi: 10.1016/s0960-9822(02)01429-x. Curr Biol. 2003. PMID: 12546806
-
Wnts as retrograde signals for axon and growth cone differentiation.Cell. 2000 Mar 3;100(5):495-7. doi: 10.1016/s0092-8674(00)80685-6. Cell. 2000. PMID: 10721986 Review. No abstract available.
-
The dynamic organizer.Nat Cell Biol. 1999 Nov;1(7):E179-81. doi: 10.1038/15616. Nat Cell Biol. 1999. PMID: 10559998 No abstract available.
-
Signalling and endocytosis: Wnt breaks down on back roads.Nat Cell Biol. 2001 Aug;3(8):E185-6. doi: 10.1038/35087126. Nat Cell Biol. 2001. PMID: 11483976 Review. No abstract available.
-
Wnts as morphogens? The view from the wing of Drosophila.Nat Rev Mol Cell Biol. 2003 Apr;4(4):321-5. doi: 10.1038/nrm1078. Nat Rev Mol Cell Biol. 2003. PMID: 12671654 Review.
Cited by
-
The DAF-7/TGF-β signaling pathway regulates abundance of the Caenorhabditis elegans glutamate receptor GLR-1.Mol Cell Neurosci. 2015 Jul;67:66-74. doi: 10.1016/j.mcn.2015.06.003. Epub 2015 Jun 5. Mol Cell Neurosci. 2015. PMID: 26054666 Free PMC article.
-
[Research progress on the role of thrombospondin in synapse formation].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019 Jan 15;33(1):124-128. doi: 10.7507/1002-1892.201809006. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019. PMID: 30644272 Free PMC article. Review. Chinese.
-
The atypical cadherin flamingo regulates synaptogenesis and helps prevent axonal and synaptic degeneration in Drosophila.Mol Cell Neurosci. 2007 Apr;34(4):662-78. doi: 10.1016/j.mcn.2007.01.007. Epub 2007 Jan 25. Mol Cell Neurosci. 2007. PMID: 17321750 Free PMC article.
-
Plasticity and second messengers during synapse development.Int Rev Neurobiol. 2006;75:237-65. doi: 10.1016/S0074-7742(06)75011-5. Int Rev Neurobiol. 2006. PMID: 17137931 Free PMC article. Review.
-
Emerging evidence for astrocyte dysfunction in schizophrenia.Glia. 2022 Sep;70(9):1585-1604. doi: 10.1002/glia.24221. Epub 2022 May 30. Glia. 2022. PMID: 35634946 Free PMC article. Review.
References
-
- Poo MM. Neurotrophins as synaptic modulators. Nature Rev. Neurosci. 2001;2:24–32. - PubMed
-
-
Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. An important early account of the involvement of postsynaptic neurotransmitter receptors in retrograde signalling at
Drosophila synapses. This article established that presynaptic transmitter release could be affected by regulating the size of postsynaptic responses.
-
-
- Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. - PubMed
-
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

