Regulation of supply and demand for maternal nutrients in mammals by imprinted genes
- PMID: 12562908
- PMCID: PMC2342627
- DOI: 10.1113/jphysiol.2002.033274
Regulation of supply and demand for maternal nutrients in mammals by imprinted genes
Abstract
The placenta has evolved in eutherian mammals primarily to provide nutrients for the developing fetus. The genetic control of the regulation of supply and demand for maternal nutrients is not understood. In this review we argue that imprinted genes have central roles in controlling both the fetal demand for, and the placental supply of, maternal nutrients. Recent studies on Igf2 (insulin-like growth factor 2) knockout mouse models provide experimental support for this hypothesis. These show effects on placental transport capacity consistent with a role of IGF-II in modulating both the placental supply and fetal demand for nutrients. Imprinting of genes with such functions may have coevolved with the placenta and new evidence suggests that transporter proteins, as well as the regulators themselves, may also be imprinted. These data and hypotheses are important, as deregulation of supply and demand affects fetal growth and has long term consequences for health in mammals both in the neonatal period and, as a result of fetal programming, in adulthood.
Figures
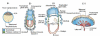
Similar articles
-
Placental adaptations to the maternal-fetal environment: implications for fetal growth and developmental programming.Reprod Biomed Online. 2012 Jul;25(1):68-89. doi: 10.1016/j.rbmo.2012.03.017. Epub 2012 Apr 5. Reprod Biomed Online. 2012. PMID: 22560117 Review.
-
Placental-specific IGF-II is a major modulator of placental and fetal growth.Nature. 2002 Jun 27;417(6892):945-8. doi: 10.1038/nature00819. Nature. 2002. PMID: 12087403
-
Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems.Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):19219-24. doi: 10.1073/pnas.0504468103. Epub 2005 Dec 19. Proc Natl Acad Sci U S A. 2005. PMID: 16365304 Free PMC article.
-
In-utero stress and mode of conception: impact on regulation of imprinted genes, fetal development and future health.Hum Reprod Update. 2019 Nov 5;25(6):777-801. doi: 10.1093/humupd/dmz025. Hum Reprod Update. 2019. PMID: 31633761
-
Regulation of placental efficiency for nutrient transport by imprinted genes.Placenta. 2006 Apr;27 Suppl A:S98-102. doi: 10.1016/j.placenta.2005.12.008. Epub 2006 Feb 28. Placenta. 2006. PMID: 16503350 Review.
Cited by
-
Role of the 820 A/G variant in the IGF-2 gene and recurrent spontaneous abortion in southern Iran: A cross-sectional study.Int J Reprod Biomed. 2020 Sep 20;18(9):747-754. doi: 10.18502/ijrm.v13i9.7669. eCollection 2020 Sep. Int J Reprod Biomed. 2020. PMID: 33062920 Free PMC article.
-
Hypothalamic expression of Peg3 gene is associated with maternal care differences between SM/J and LG/J mouse strains.Brain Behav. 2012 Jul;2(4):365-76. doi: 10.1002/brb3.58. Brain Behav. 2012. PMID: 22950040 Free PMC article.
-
Placental adaptive responses and fetal programming.J Physiol. 2006 Apr 1;572(Pt 1):25-30. doi: 10.1113/jphysiol.2006.104968. Epub 2006 Feb 9. J Physiol. 2006. PMID: 16469781 Free PMC article. Review.
-
Developmental origin of health and disease.J Physiol. 2006 Apr 1;572(Pt 1):3-4. doi: 10.1113/jphysiol.2006.107680. Epub 2006 Feb 23. J Physiol. 2006. PMID: 16497709 Free PMC article. No abstract available.
-
Limited evolutionary conservation of imprinting in the human placenta.Proc Natl Acad Sci U S A. 2006 Apr 25;103(17):6623-8. doi: 10.1073/pnas.0511031103. Epub 2006 Apr 13. Proc Natl Acad Sci U S A. 2006. PMID: 16614068 Free PMC article.
References
-
- Alleman M, Doctor J. Genomic imprinting in plants: observations and evolutionary implications. Plant Mol Biol. 2000;43:147–161. - PubMed
-
- Ayuk PT, Theophanous D, D'Souza SW, Sibley CP, Glazier JD. L-arginine transport by the microvillous plasma membrane of the syncytiotrophoblast from human placenta in relation to nitric oxide production: effects of gestation, preeclampsia, and intrauterine growth restriction. J Clin Endocrinol Metab. 2002;87:747–751. - PubMed
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. - PubMed
-
- Barker DJP. Fetal programming and public health. In: O'Brien PMS, Wheeler T, Barker DJP, editors. Fetal Programming: Influences on Development and Disease in Later Life. London: RCOG Press; 1999. pp. 3–11.
-
- Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103–121. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

