The amino-terminal extensions of the longer Sendai virus C proteins modulate pY701-Stat1 and bulk Stat1 levels independently of interferon signaling
- PMID: 12551969
- PMCID: PMC141115
- DOI: 10.1128/jvi.77.4.2321-2329.2003
The amino-terminal extensions of the longer Sendai virus C proteins modulate pY701-Stat1 and bulk Stat1 levels independently of interferon signaling
Abstract
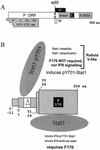
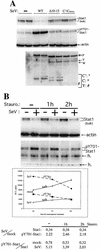
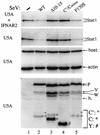
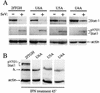
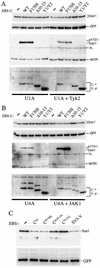
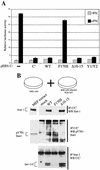
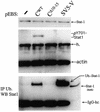
The Sendai virus (SeV) C proteins are known to interact with Stat1 to prevent interferon (IFN)-induced pY701-Stat1 formation and IFN signaling. Nevertheless, pY701-Stat1 levels paradoxically increase during SeV infection. The C proteins also induce bulk Stat1 instability in some cells, similar to rubulavirus V proteins. We have found that SeV infection increases pY701-Stat1 levels even in cells in which bulk Stat1 levels strongly decrease. Remarkably, both the decrease in bulk Stat1 levels and the increase in pY701-Stat1 levels were found to be independent of the IFN signaling system, i.e., these events occur in mutant cells in which various components of the IFN signaling system have been disabled. Consistent with this, the C-induced decrease in Stat1 levels does not require Y701 of Stat1. We present evidence that C interacts with Stat1 in two different ways, one that prevents IFN-induced pY701-Stat1 formation and IFN signaling that has already been documented, and another that induces pY701-Stat1 formation (while decreasing bulk Stat1 levels) in a manner that does not require IFN signaling. These two types of Stat1 interaction are also distinguishable by C gene mutations. In particular, the IFN signaling-independent Stat1 interactions specifically require the amino-terminal extensions of the longer C proteins. The actions of the SeV C proteins in counteracting the cellular antiviral response are clearly more extensive than previously appreciated.
Figures

Similar articles
-
All four Sendai Virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation.Virology. 2002 Apr 10;295(2):256-65. doi: 10.1006/viro.2001.1342. Virology. 2002. PMID: 12033784
-
Longer and shorter forms of Sendai virus C proteins play different roles in modulating the cellular antiviral response.J Virol. 2001 Aug;75(15):6800-7. doi: 10.1128/JVI.75.15.6800-6807.2001. J Virol. 2001. PMID: 11435558 Free PMC article.
-
Characterization of the amino acid residues of sendai virus C protein that are critically involved in its interferon antagonism and RNA synthesis down-regulation.J Virol. 2004 Jul;78(14):7443-54. doi: 10.1128/JVI.78.14.7443-7454.2004. J Virol. 2004. PMID: 15220418 Free PMC article.
-
[Sendai virus proteins counteracting the host innate immunity].Uirusu. 2004 Dec;54(2):179-88. doi: 10.2222/jsv.54.179. Uirusu. 2004. PMID: 15745155 Review. Japanese.
-
Paramyxovirus strategies for evading the interferon response.Rev Med Virol. 2002 Nov-Dec;12(6):337-57. doi: 10.1002/rmv.357. Rev Med Virol. 2002. PMID: 12410527 Review.
Cited by
-
Human parainfluenza virus type 1 C proteins are nonessential proteins that inhibit the host interferon and apoptotic responses and are required for efficient replication in nonhuman primates.J Virol. 2008 Sep;82(18):8965-77. doi: 10.1128/JVI.00853-08. Epub 2008 Jul 9. J Virol. 2008. PMID: 18614629 Free PMC article.
-
Paramyxovirus evasion of innate immunity: Diverse strategies for common targets.World J Virol. 2013 May 12;2(2):57-70. doi: 10.5501/wjv.v2.i2.57. World J Virol. 2013. PMID: 24175230 Free PMC article. Review.
-
Generation of recombinant human parainfluenza virus type 1 vaccine candidates by importation of temperature-sensitive and attenuating mutations from heterologous paramyxoviruses.J Virol. 2004 Feb;78(4):2017-28. doi: 10.1128/jvi.78.4.2017-2028.2004. J Virol. 2004. PMID: 14747566 Free PMC article.
-
Targeting of the Sendai virus C protein to the plasma membrane via a peptide-only membrane anchor.J Virol. 2007 Apr;81(7):3187-97. doi: 10.1128/JVI.02465-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229713 Free PMC article.
-
Evolution and structural organization of the C proteins of paramyxovirinae.PLoS One. 2014 Feb 25;9(2):e90003. doi: 10.1371/journal.pone.0090003. eCollection 2014. PLoS One. 2014. PMID: 24587180 Free PMC article.
References
-
- Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
-
- Chatterjee-Kishore, M., A. F. van Den, and G. R. Stark. 2000. Adenovirus E1A down-regulates LMP2 transcription by interfering with the binding of stat1 to IRF1. J. Biol. Chem. 275:20406-20411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

