Pharmacokinetics of diclofenac and inhibition of cyclooxygenases 1 and 2: no relationship to the CYP2C9 genetic polymorphism in humans
- PMID: 12534640
- PMCID: PMC1884192
- DOI: 10.1046/j.1365-2125.2003.01712.x
Pharmacokinetics of diclofenac and inhibition of cyclooxygenases 1 and 2: no relationship to the CYP2C9 genetic polymorphism in humans
Abstract
Aims: The cytochrome P450 enzyme CYP2C9 catalyses the 4'-hydroxylation of the nonsteroidal analgesic drug diclofenac in humans. We studied the influences of the known amino acid variants, CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu), on diclofenac pharmacokinetics after a 50-mg oral dose of diclofenac in healthy volunteers. As a surrogate marker of diclofenac activity, the ex vivo formation of prostaglandin E2 and thromboxane B2, which reflects COX-2 and COX-1 activity, was measured.
Methods: Genotyping was performed in 516 healthy volunteers to obtain 20 participants with all allelic combinations of the two CYP2C9 variants Arg144Cys (*2) and Ile359Leu (*3). Diclofenac and 4'-hydroxydiclofenac were quantified in plasma by reversed phase h.p.l.c. after oral intake of 50 mg diclofenac. Concentrations of thromboxane B2 (TxB2) and prostaglandin E2 (PGE2) were measured by immunoassays.
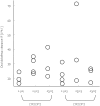
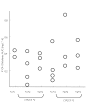
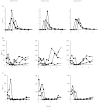
Results: There was no evidence of impaired metabolism of oral diclofenac in heterozygous and homozygous carriers of the CYP2C9 alleles *2 and *3 compared with the wild type (mean CL/F (95% CI) 20.5 (11, 30) l h-1 for *1/*1, 29.9 (19, 40) l h-1 for *1/*2, 30.0 (4, 56) l h-1 for *2/*2, 22.6 (12, 33) l h-1 for *1/*3, 23.5 (11, 37) l h-1 for *3/*3 and 37.3 (-15, 89) l h-1 in *2/*3). Furthermore, plasma concentrations of the metabolite 4'-hydroxydiclofenac were not lower in carriers of the CYP2C9 low-activity alleles *2 and *3 compared with carriers of the CYP2C9*1/*1 genotype. Marked diclofenac mediated inhibition of COX-1- and COX-2 activity was detected in all individuals independent of CYP2C9 genotype.
Conclusions: Polymorphisms of the CYP2C9 gene had no discernible effect on the pharmacokinetics and pharmacodynamics of diclofenac. The question of whether enzymes other than CYP2C9 play a major role in diclofenac 4'-hydroxylation in vivo or whether 4'-hydroxylation is not a rate-limiting step in diclofenac elimination in vivo, or whether the effect of the CYP2C9 polymorphisms is substrate-dependent, needs further investigation.
Figures
Similar articles
-
Influence of age and cytochrome P450 2C9 genotype on the steady-state disposition of diclofenac and celecoxib.Clin Pharmacokinet. 2003;42(3):283-92. doi: 10.2165/00003088-200342030-00003. Clin Pharmacokinet. 2003. PMID: 12603175 Clinical Trial.
-
Enantiospecific effects of cytochrome P450 2C9 amino acid variants on ibuprofen pharmacokinetics and on the inhibition of cyclooxygenases 1 and 2.Clin Pharmacol Ther. 2002 Jul;72(1):62-75. doi: 10.1067/mcp.2002.125726. Clin Pharmacol Ther. 2002. PMID: 12152005 Clinical Trial.
-
In-vitro metabolism of celecoxib, a cyclooxygenase-2 inhibitor, by allelic variant forms of human liver microsomal cytochrome P450 2C9: correlation with CYP2C9 genotype and in-vivo pharmacokinetics.Pharmacogenetics. 2001 Apr;11(3):223-35. doi: 10.1097/00008571-200104000-00006. Pharmacogenetics. 2001. PMID: 11337938
-
Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor.Clin Pharmacokinet. 2000 Mar;38(3):225-42. doi: 10.2165/00003088-200038030-00003. Clin Pharmacokinet. 2000. PMID: 10749518 Review.
-
Impact of CYP2C9 genotype on pharmacokinetics: are all cyclooxygenase inhibitors the same?Drug Metab Dispos. 2005 Nov;33(11):1567-75. doi: 10.1124/dmd.105.006452. Epub 2005 Aug 23. Drug Metab Dispos. 2005. PMID: 16118328 Review.
Cited by
-
Development and Validation of an HPLC-UV Method for the Quantification of 4'-Hydroxydiclofenac Using Salicylic Acid: Future Applications for Measurement of In Vitro Drug-Drug Interaction in Rat Liver Microsomes.Molecules. 2022 Jun 2;27(11):3587. doi: 10.3390/molecules27113587. Molecules. 2022. PMID: 35684519 Free PMC article.
-
Drug-drug-gene interactions as mediators of adverse drug reactions to diclofenac and statins: a case report and literature review.Arh Hig Rada Toksikol. 2021 Jun 28;72(3):114-128. doi: 10.2478/aiht-2021-72-3549. Arh Hig Rada Toksikol. 2021. PMID: 34187111 Free PMC article. Review.
-
Pharmacogenetics: data, concepts and tools to improve drug discovery and drug treatment.Eur J Clin Pharmacol. 2008 Feb;64(2):133-57. doi: 10.1007/s00228-007-0424-z. Epub 2008 Jan 26. Eur J Clin Pharmacol. 2008. PMID: 18224312 Free PMC article. Review.
-
[Does genomics determine efficacy of analgesics?].Schmerz. 2005 Oct;19(5):372-7. doi: 10.1007/s00482-005-0422-y. Schmerz. 2005. PMID: 16096768 German.
-
Current evidence for a modulation of low back pain by human genetic variants.J Cell Mol Med. 2009 Aug;13(8B):1605-1619. doi: 10.1111/j.1582-4934.2009.00703.x. Epub 2009 Feb 17. J Cell Mol Med. 2009. PMID: 19228264 Free PMC article. Review.
References
-
- Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diclofenac sodium: a review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs. 1980;20:24–48. - PubMed
-
- O'Brien WM. Adverse reactions to nonsteroidal anti-inflammatory drugs. Diclofenac compared with other nonsteroidal anti-inflammatory drugs. Am J Med. 1986;80:70–80. - PubMed
-
- Stierlin H, Faigle JW. Biotransformation of diclofenac sodium (Voltaren) in animals and in man. II. Quantitative determination of the unchanged drug and principal phenolic metabolites, in urine and bile. Xenobiotica. 1979;9:611–621. - PubMed
-
- Stierlin H, Faigle JW, Sallmann A, et al. Biotransformation of diclofenac sodium (Voltaren) in animals and in man. I. Isolation and identification of principal metabolites. Xenobiotica. 1979;9:601–610. - PubMed
-
- Tang W, Stearns RA, Wang RW, Chiu SH, Baillie TA. Roles of human hepatic cytochrome P450s 2C9 and 3A4 in the metabolic activation of diclofenac. Chem Res Toxicol. 1999;12:192–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

