Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae
- PMID: 12529432
- PMCID: PMC140233
- DOI: 10.1091/mbc.e02-08-0483
Piecemeal microautophagy of nucleus in Saccharomyces cerevisiae
Abstract
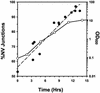
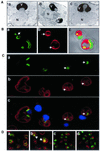
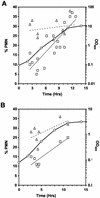
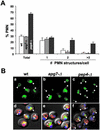
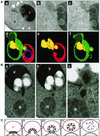
Nucleus-vacuole (NV) junctions in Saccharomyces cerevisiae are formed through specific interactions between Vac8p on the vacuole membrane and Nvj1p in the nuclear envelope. Herein, we report that NV junctions in yeast promote piecemeal microautophagy of the nucleus (PMN). During PMN, teardrop-like blebs are pinched from the nucleus, released into the vacuole lumen, and degraded by soluble hydrolases. PMN occurs in rapidly dividing cells but is induced to higher levels by carbon and nitrogen starvation and is under the control of the Tor kinase nutrient-sensing pathway. Confocal and biochemical assays demonstrate that Nvj1p is degraded in a PMN-dependent manner. PMN occurs normally in apg7-delta cells and is, therefore, not dependent on macroautophagy. Transmission electron microscopy reveals that portions of the granular nucleolus are often sequestered into PMN structures. These results introduce a novel mode of selective microautophagy that targets nonessential components of the yeast nucleus for degradation and recycling in the vacuole.
Figures


Similar articles
-
Nvj1p is the outer-nuclear-membrane receptor for oxysterol-binding protein homolog Osh1p in Saccharomyces cerevisiae.J Cell Sci. 2004 Oct 1;117(Pt 21):4959-68. doi: 10.1242/jcs.01372. Epub 2004 Sep 14. J Cell Sci. 2004. PMID: 15367582
-
Targeting of Tsc13p to nucleus-vacuole junctions: a role for very-long-chain fatty acids in the biogenesis of microautophagic vesicles.Mol Biol Cell. 2005 Sep;16(9):3987-98. doi: 10.1091/mbc.e05-04-0290. Epub 2005 Jun 15. Mol Biol Cell. 2005. PMID: 15958487 Free PMC article.
-
Nucleus-vacuole junctions and piecemeal microautophagy of the nucleus in S. cerevisiae.Autophagy. 2007 Mar-Apr;3(2):85-92. doi: 10.4161/auto.3586. Epub 2007 Mar 2. Autophagy. 2007. PMID: 17204844 Review.
-
Nucleus-vacuole junctions in Saccharomyces cerevisiae are formed through the direct interaction of Vac8p with Nvj1p.Mol Biol Cell. 2000 Jul;11(7):2445-57. doi: 10.1091/mbc.11.7.2445. Mol Biol Cell. 2000. PMID: 10888680 Free PMC article.
-
Nucleus-vacuole junctions in yeast: anatomy of a membrane contact site.Biochem Soc Trans. 2006 Jun;34(Pt 3):340-2. doi: 10.1042/BST0340340. Biochem Soc Trans. 2006. PMID: 16709156 Review.
Cited by
-
Lipid Exchangers: Cellular Functions and Mechanistic Links With Phosphoinositide Metabolism.Front Cell Dev Biol. 2020 Jul 21;8:663. doi: 10.3389/fcell.2020.00663. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32793602 Free PMC article. Review.
-
The many faces of mitochondrial autophagy: making sense of contrasting observations in recent research.Int J Cell Biol. 2012;2012:431684. doi: 10.1155/2012/431684. Epub 2012 Apr 8. Int J Cell Biol. 2012. PMID: 22550491 Free PMC article.
-
Membrane Contact Sites in Autophagy.Cells. 2022 Nov 28;11(23):3813. doi: 10.3390/cells11233813. Cells. 2022. PMID: 36497073 Free PMC article. Review.
-
Cdc48/p97 and Shp1/p47 regulate autophagosome biogenesis in concert with ubiquitin-like Atg8.J Cell Biol. 2010 Sep 20;190(6):965-73. doi: 10.1083/jcb.201002075. J Cell Biol. 2010. PMID: 20855502 Free PMC article.
-
Comparative genomics of nuclear envelope proteins.BMC Genomics. 2018 Nov 16;19(1):823. doi: 10.1186/s12864-018-5218-4. BMC Genomics. 2018. PMID: 30445911 Free PMC article.
References
-
- Achleitner G, Gaigg B, Krasser A, Kainersdorfer E, Kohlwein SD, Perktold A, Zellnig G, Daum G. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur J Biochem. 1999;264:545–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

