Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants
- PMID: 12525653
- PMCID: PMC140920
- DOI: 10.1128/jvi.77.3.2182-2194.2003
Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants
Abstract
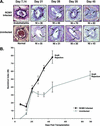
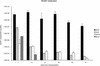
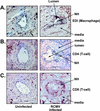
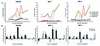
Cytomegalovirus (CMV) infections have been shown to dramatically affect solid organ transplant graft survival in both human and animal models. Recently, it was demonstrated that rat CMV (RCMV) infection accelerates the development of transplant vascular sclerosis (TVS) in both rat heart and small bowel graft transplants. However, the mechanisms involved in this process are still unclear. In the present study, we determined the kinetics of RCMV-accelerated TVS in a rat heart transplant model. Acute RCMV infection enhances the development of TVS in rat heart allografts, and this process is initiated between 21 and 24 days posttransplantation. The virus is consistently detected in the heart grafts from day 7 until day 35 posttransplantation but is rarely found at the time of graft rejection (day 45 posttransplantation). Grafts from RCMV-infected recipients had upregulation of chemokine expression compared to uninfected controls, and the timing of this increased expression paralleled that of RCMV-accelerated neointimal formation. In addition, graft vessels from RCMV-infected grafts demonstrate the increased infiltration of T cells and macrophages during periods of highest chemokine expression. These results suggest that CMV-induced acceleration of TVS involves the increased graft vascular infiltration of inflammatory cells through enhanced chemokine expression.
Figures
Similar articles
-
Cytomegalovirus accelerates chronic allograft nephropathy in a rat renal transplant model with associated provocative chemokine profiles.Transplant Proc. 2006 Dec;38(10):3214-20. doi: 10.1016/j.transproceed.2006.10.187. Transplant Proc. 2006. PMID: 17175227
-
Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants.Transplantation. 2002 Mar 15;73(5):679-88. doi: 10.1097/00007890-200203150-00005. Transplantation. 2002. PMID: 11907411
-
Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33.Am J Transplant. 2005 Mar;5(3):436-42. doi: 10.1111/j.1600-6143.2004.00711.x. Am J Transplant. 2005. PMID: 15707397
-
Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing.Curr Top Microbiol Immunol. 2008;325:397-415. doi: 10.1007/978-3-540-77349-8_22. Curr Top Microbiol Immunol. 2008. PMID: 18637518 Free PMC article. Review.
-
Chemokines: what chemokine is that?Curr Biol. 1997 Jun 1;7(6):R384-6. doi: 10.1016/s0960-9822(06)00181-3. Curr Biol. 1997. PMID: 9197234 Review.
Cited by
-
Innate pathways of immune activation in transplantation.J Transplant. 2010;2010:826240. doi: 10.1155/2010/826240. Epub 2010 Aug 31. J Transplant. 2010. PMID: 20871653 Free PMC article.
-
IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin.Blood. 2011 Jan 6;117(1):352-61. doi: 10.1182/blood-2010-06-291245. Epub 2010 Oct 7. Blood. 2011. PMID: 20930069 Free PMC article.
-
Cytomegalovirus microRNA expression is tissue specific and is associated with persistence.J Virol. 2011 Jan;85(1):378-89. doi: 10.1128/JVI.01900-10. Epub 2010 Oct 27. J Virol. 2011. PMID: 20980502 Free PMC article.
-
Rat cytomegalovirus gene expression in cardiac allograft recipients is tissue specific and does not parallel the profiles detected in vitro.J Virol. 2007 Apr;81(8):3816-26. doi: 10.1128/JVI.02425-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251289 Free PMC article.
-
Cytomegalovirus latency promotes cardiac lymphoid neogenesis and accelerated allograft rejection in CMV naïve recipients.Am J Transplant. 2011 Jan;11(1):45-55. doi: 10.1111/j.1600-6143.2010.03365.x. Am J Transplant. 2011. PMID: 21199347 Free PMC article.
References
-
- Adler, S. P. 1983. Transfusion-associated cytomegalovirus infections. Rev. Infect. Dis. 5:977-993. - PubMed
-
- Armstrong, A. T., A. R. Strauch, R. C. Starling, D. D. Sedmak, and C. G. Orosz. 1997. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplantation 63:941-947. - PubMed
-
- Ballard, R. A., W. L. Drew, K. G. Hufnagle, and P. A. Riedel. 1979. Acquired cytomegalovirus infection in preterm infants. Am. J. Dis. Child. 133:482-485. - PubMed
-
- Boehler, A., A. Schaffner, F. Salomon, and G. Keusch. 1994. Cytomegalovirus disease of late onset following renal transplantation: a potentially fatal entity. Scand. J. Infect. Dis. 26:369-373. - PubMed
-
- Bruggeman, C. A., H. Meijer, F. Bosman, and C. P. van Boven. 1985. Biology of rat cytomegalovirus infection. Intervirology 24:1-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

