Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover
- PMID: 12524413
- PMCID: PMC1774949
- DOI: 10.1136/gut.52.2.275
Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover
Abstract
Background: Pancreatic fibrosis is a characteristic feature of chronic pancreatic injury and is thought to result from a change in the balance between synthesis and degradation of extracellular matrix (ECM) proteins. Recent studies suggest that activated pancreatic stellate cells (PSCs) play a central role in pancreatic fibrogenesis via increased synthesis of ECM proteins. However, the role of these cells in ECM protein degradation has not been fully elucidated.
Aims: To determine: (i) whether PSCs secrete matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) and, if so (ii) whether MMP and TIMP secretion by PSCs is altered in response to known PSC activating factors such as tumour necrosis factor alpha (TNF-alpha), transforming growth factor beta1 (TGF-beta1), interleukin 6 (IL-6), ethanol, and acetaldehyde.
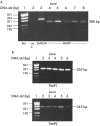
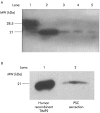
Methods: Cultured rat PSCs (n=3-5 separate cell preparations) were incubated at 37 degrees C for 24 hours with serum free culture medium containing TNF-alpha (5-25 U/ml), TGF-beta1 (0.5-1 ng/ml), IL-6 (0.001-10 ng/ml), ethanol (10-50 mM), or acetaldehyde (150-200 micro M), or no additions (controls). Medium from control cells was examined for the presence of MMPs by zymography using a 10% polyacrylamide-0.1% gelatin gel. Reverse transcriptase-polymerase chain reaction (RT-PCR) was used to examine gene expression of MMP9 and the tissue inhibitors of metalloproteinases TIMP1 and TIMP2. Western blotting was used to identify a specific MMP, MMP2 (a gelatinase that digests basement membrane collagen and the dominant MMP observed on zymography) and a specific TIMP, TIMP2. Reverse zymography was used to examine functional TIMPs in PSC secretions. The effect of TNF-alpha, TGF-beta1, and IL-6 on MMP2 secretion was assessed by densitometry of western blots. The effect of ethanol and acetaldehyde on MMP2 and TIMP2 secretion was also assessed by this method.
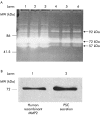
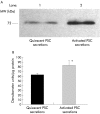
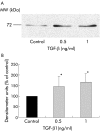
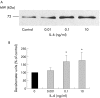
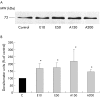
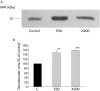
Results: Zymography revealed that PSCs secrete a number of MMPs including proteinases with molecular weights consistent with MMP2, MMP9, and MMP13. RT-PCR demonstrated the presence of mRNA for metalloproteinase inhibitors TIMP1 and TIMP2 in PSCs while reverse zymography revealed the presence of functional TIMP2 in PSC secretions. MMP2 secretion by PSCs was significantly increased by TGF-beta1 and IL-6, but was not affected by TNF-alpha. Ethanol and acetaldehyde induced secretion of both MMP2 and TIMP2 by PSCs.
Conclusions: Pancreatic stellate cells have the capacity to synthesise a number of matrix metalloproteinases, including MMP2, MMP9, and MMP13 and their inhibitors TIMP1 and TIMP2. MMP2 secretion by PSCs is significantly increased on exposure to the proinflammatory cytokines TGF-beta1 and IL-6. Both ethanol and its metabolite acetaldehyde increase MMP2 as well as TIMP2 secretion by PSCs.
Implication: The role of pancreatic stellate cells in extracellular matrix formation and fibrogenesis may be related to their capacity to regulate the degradation as well as the synthesis of extracellular matrix proteins.
Figures
Similar articles
-
Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart.J Pathol. 2001 Jun;194(2):225-31. doi: 10.1002/path.850. J Pathol. 2001. PMID: 11400152
-
Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis.Am J Pathol. 2002 May;160(5):1787-98. doi: 10.1016/s0002-9440(10)61125-x. Am J Pathol. 2002. PMID: 12000730 Free PMC article.
-
Elevated ratio of MMP2/MMP9 activity is associated with poor response to chemotherapy in osteosarcoma.BMC Cancer. 2016 Mar 15;16:223. doi: 10.1186/s12885-016-2266-5. BMC Cancer. 2016. PMID: 26979530 Free PMC article.
-
The Biology and Function of Tissue Inhibitor of Metalloproteinase 2 in the Lungs.Pulm Med. 2022 Dec 31;2022:3632764. doi: 10.1155/2022/3632764. eCollection 2022. Pulm Med. 2022. PMID: 36624735 Free PMC article. Review.
-
The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases.Int J Mol Sci. 2020 Dec 20;21(24):9739. doi: 10.3390/ijms21249739. Int J Mol Sci. 2020. PMID: 33419373 Free PMC article. Review.
Cited by
-
Role of Plasma MMP 9 levels in the Pathogenesis of Chronic Pancreatitis.Indian J Clin Biochem. 2011 Apr;26(2):136-9. doi: 10.1007/s12291-010-0103-1. Epub 2011 Jan 8. Indian J Clin Biochem. 2011. PMID: 22468039 Free PMC article.
-
Noninvasive Assessment of Liver Fibrosis: Current and Future Clinical and Molecular Perspectives.Int J Mol Sci. 2020 Jul 11;21(14):4906. doi: 10.3390/ijms21144906. Int J Mol Sci. 2020. PMID: 32664553 Free PMC article. Review.
-
PD98059 inhibited the activation of pancreatic stellate cells mediated by platelet-derived growth factor BB in rats.J Huazhong Univ Sci Technolog Med Sci. 2005;25(3):297-9, 306. doi: 10.1007/BF02828148. J Huazhong Univ Sci Technolog Med Sci. 2005. PMID: 16201277
-
Pancreatic Stellate Cells Prolong Ex Vivo Islet Viability and Function and Improve Engraftment.Stem Cells Transl Med. 2022 Jun 22;11(6):630-643. doi: 10.1093/stcltm/szac018. Stem Cells Transl Med. 2022. PMID: 35438788 Free PMC article.
-
TRPM7 Modulates Human Pancreatic Stellate Cell Activation.Cells. 2022 Jul 21;11(14):2255. doi: 10.3390/cells11142255. Cells. 2022. PMID: 35883700 Free PMC article.
References
-
- Apte MV, Phillips PA, Fahmy RG, et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology 2000;118:780–94. - PubMed
-
- Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
