Heart failure -- a challenge to our current concepts of excitation-contraction coupling
- PMID: 12509477
- PMCID: PMC2342477
- DOI: 10.1113/jphysiol.2002.034728
Heart failure -- a challenge to our current concepts of excitation-contraction coupling
Abstract
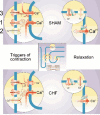
Development of novel therapeutic strategies for congestive heart failure (CHF) seems to be hampered by insufficient knowledge of the molecular machinery of excitation-contraction (EC) coupling in both normal and failing hearts. Cardiac hypertrophy and failure represent a multitude of cardiac phenotypes, and available invasive and non-invasive techniques, briefly reviewed here, allow proper quantification of myocardial function in experimental models even in rats and mice. Both reduced fractional shortening and reduced velocity of contraction characterize myocardial failure. Only when myocardial function is depressed in vivo can meaningful studies be done in vitro of contractility and EC coupling. Also, we point out potential limitations with the whole cell patch clamp technique. Two main factors stand out as explanations for myocardial failure. First, a basic feature of CHF seems to be a reduced Ca(2+) load of the sarcoplasmic reticulum (SR) mainly due to a low phosphorylation level of phospholamban. Second, there seems to be a defect of the trigger mechanism of Ca(2+) release from the SR. We argue that this defect only becomes manifest in the presence of reduced Ca(2+) reuptake capacity of the SR and that it may not be solely attributable to reduced gain of the Ca(2+)-induced Ca(2+) release (CICR). We list several possible explanations for this defect that represent important avenues for future research.
Figures

Similar articles
-
Defective excitation-contraction coupling in hearts of rats with congestive heart failure.Acta Physiol Scand. 2005 May;184(1):45-58. doi: 10.1111/j.1365-201X.2005.01431.x. Acta Physiol Scand. 2005. PMID: 15847643
-
Ca flux, contractility, and excitation-contraction coupling in hypertrophic rat ventricular myocytes.Am J Physiol. 1998 Apr;274(4):H1348-60. doi: 10.1152/ajpheart.1998.274.4.H1348. Am J Physiol. 1998. PMID: 9575940
-
Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure.Circ Arrhythm Electrophysiol. 2008 Jun 1;1(2):93-102. doi: 10.1161/CIRCEP.107.754788. Epub 2008 Apr 30. Circ Arrhythm Electrophysiol. 2008. PMID: 19808399
-
Cardiac sodium transport and excitation-contraction coupling.J Mol Cell Cardiol. 2013 Aug;61:11-9. doi: 10.1016/j.yjmcc.2013.06.003. Epub 2013 Jun 14. J Mol Cell Cardiol. 2013. PMID: 23774049 Review.
-
Abnormalities of calcium cycling in the hypertrophied and failing heart.J Mol Cell Cardiol. 2000 Sep;32(9):1595-607. doi: 10.1006/jmcc.2000.1206. J Mol Cell Cardiol. 2000. PMID: 10966823 Review.
Cited by
-
Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure.Circulation. 2011 May 3;123(17):1881-90. doi: 10.1161/CIRCULATIONAHA.110.989707. Epub 2011 Apr 18. Circulation. 2011. PMID: 21502574 Free PMC article.
-
The L-type calcium channel in the heart: the beat goes on.J Clin Invest. 2005 Dec;115(12):3306-17. doi: 10.1172/JCI27167. J Clin Invest. 2005. PMID: 16322774 Free PMC article. Review.
-
Arrhythmogenic and metabolic remodelling of failing human heart.J Physiol. 2016 Jul 15;594(14):3963-80. doi: 10.1113/JP271992. Epub 2016 Jun 12. J Physiol. 2016. PMID: 27019074 Free PMC article. Review.
-
Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes.Am J Physiol Heart Circ Physiol. 2009 Dec;297(6):H1992-2003. doi: 10.1152/ajpheart.00602.2009. Epub 2009 Sep 25. Am J Physiol Heart Circ Physiol. 2009. PMID: 19783774 Free PMC article.
-
Enhanced basal contractility but reduced excitation-contraction coupling efficiency and beta-adrenergic reserve of hearts with increased Cav1.2 activity.Am J Physiol Heart Circ Physiol. 2010 Aug;299(2):H519-28. doi: 10.1152/ajpheart.00265.2010. Epub 2010 Jun 11. Am J Physiol Heart Circ Physiol. 2010. PMID: 20543081 Free PMC article.
References
-
- Alpert NR, Mulieri LA, Warshaw D. The failing human heart. Cardiovasc Res. 2002;54:1–10. - PubMed
-
- Anand IS, Liu D, Chugh SS, Prahash AJ, Gupta S, John R, Popescu F, Chandrashekhar Y. Isolated myocyte contractile function is normal in postinfarct remodeled rat heart with systolic dysfunction. Circulation. 1997;96:3974–3984. - PubMed
-
- Baker AJ, Figueredo VM, Keung EC, Camacho SA. Ca2+ regulates the kinetics of tension development in intact cardiac muscle. Am J Physiol. 1998;44:H744–750. - PubMed
-
- Barrere-Lemaire S, Piot C, Leclercq F, Nargeot J, Richard S. Facilitation of L-type calcium currents by diastolic depolarization in cardiac cells: impairment in heart failure. Cardiovasc Res. 2000;47:336–349. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

