Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus
- PMID: 12486245
- PMCID: PMC140946
- DOI: 10.1073/pnas.0135345100
Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus
Abstract
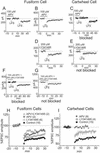
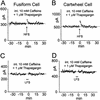
The dorsal cochlear nucleus integrates acoustic with multimodal sensory inputs from widespread areas of the brain. Multimodal inputs are brought to spiny dendrites of fusiform and cartwheel cells in the molecular layer by parallel fibers through synapses that are subject to long-term potentiation and long-term depression. Acoustic cues are brought to smooth dendrites of fusiform cells in the deep layer by auditory nerve fibers through synapses that do not show plasticity. Plasticity requires Ca(2+)-induced Ca(2+) release; its sensitivity to antagonists of N-methyl-d-aspartate and metabotropic glutamate receptors differs in fusiform and cartwheel cells.
Figures



Similar articles
-
Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus.Nat Neurosci. 2004 Jul;7(7):719-25. doi: 10.1038/nn1272. Epub 2004 Jun 20. Nat Neurosci. 2004. PMID: 15208632
-
Plasticity of synaptic GluN receptors is required for the Src-dependent induction of long-term potentiation at CA3-CA1 synapses.Hippocampus. 2011 Oct;21(10):1053-61. doi: 10.1002/hipo.20818. Epub 2010 Jun 2. Hippocampus. 2011. PMID: 20865743
-
Induction of homosynaptic long-term depression at spinal synapses of sensory a delta-fibers requires activation of metabotropic glutamate receptors.Neuroscience. 2000;98(1):141-8. doi: 10.1016/s0306-4522(00)00080-4. Neuroscience. 2000. PMID: 10858620
-
Mechanisms of synaptic plasticity in the dorsal cochlear nucleus: plasticity-induced changes that could underlie tinnitus.Am J Audiol. 2008 Dec;17(2):S170-5. doi: 10.1044/1059-0889(2008/07-0030). Epub 2008 Oct 31. Am J Audiol. 2008. PMID: 18978197 Free PMC article. Review.
-
What's a cerebellar circuit doing in the auditory system?Trends Neurosci. 2004 Feb;27(2):104-10. doi: 10.1016/j.tins.2003.12.001. Trends Neurosci. 2004. PMID: 15102490 Review.
Cited by
-
Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity.Neuron. 2007 Apr 19;54(2):291-301. doi: 10.1016/j.neuron.2007.03.026. Neuron. 2007. PMID: 17442249 Free PMC article.
-
Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation.Neuron. 2010 Nov 4;68(3):500-11. doi: 10.1016/j.neuron.2010.09.026. Neuron. 2010. PMID: 21040850 Free PMC article.
-
Activation of synaptic group II metabotropic glutamate receptors induces long-term depression at GABAergic synapses in CNS neurons.J Neurosci. 2013 Oct 2;33(40):15964-77. doi: 10.1523/JNEUROSCI.0202-13.2013. J Neurosci. 2013. PMID: 24089501 Free PMC article.
-
Anatomy and Physiology of Metabotropic Glutamate Receptors in Mammalian and Avian Auditory System.HSOA Trends Anat Physiol. 2018;1:001. doi: 10.24966/TAP-7752/100001. Epub 2018 Feb 9. HSOA Trends Anat Physiol. 2018. PMID: 30854519 Free PMC article.
-
Plasticity of somatosensory inputs to the cochlear nucleus--implications for tinnitus.Hear Res. 2011 Nov;281(1-2):38-46. doi: 10.1016/j.heares.2011.05.001. Epub 2011 May 18. Hear Res. 2011. PMID: 21620940 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

