Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo
- PMID: 12481082
- PMCID: PMC166710
- DOI: 10.1104/pp.008250
Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo
Abstract
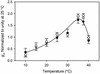
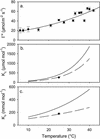
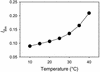
CO(2) transfer conductance from the intercellular airspaces of the leaf into the chloroplast, defined as mesophyll conductance (g(m)), is finite. Therefore, it will limit photosynthesis when CO(2) is not saturating, as in C3 leaves in the present atmosphere. Little is known about the processes that determine the magnitude of g(m). The process dominating g(m) is uncertain, though carbonic anhydrase, aquaporins, and the diffusivity of CO(2) in water have all been suggested. The response of g(m) to temperature (10 degrees C-40 degrees C) in mature leaves of tobacco (Nicotiana tabacum L. cv W38) was determined using measurements of leaf carbon dioxide and water vapor exchange, coupled with modulated chlorophyll fluorescence. These measurements revealed a temperature coefficient (Q(10)) of approximately 2.2 for g(m), suggesting control by a protein-facilitated process because the Q(10) for diffusion of CO(2) in water is about 1.25. Further, g(m) values are maximal at 35 degrees C to 37.5 degrees C, again suggesting a protein-facilitated process, but with a lower energy of deactivation than Rubisco. Using the temperature response of g(m) to calculate CO(2) at Rubisco, the kinetic parameters of Rubisco were calculated in vivo from 10 degrees C to 40 degrees C. Using these parameters, we determined the limitation imposed on photosynthesis by g(m). Despite an exponential rise with temperature, g(m) does not keep pace with increased capacity for CO(2) uptake at the site of Rubisco. The fraction of the total limitations to CO(2) uptake within the leaf attributable to g(m) rose from 0.10 at 10 degrees C to 0.22 at 40 degrees C. This shows that transfer of CO(2) from the intercellular air space to Rubisco is a very substantial limitation on photosynthesis, especially at high temperature.
Figures
Similar articles
-
Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat.Plant Physiol. 2002 Mar;128(3):1087-97. doi: 10.1104/pp.010919. Plant Physiol. 2002. PMID: 11891263 Free PMC article.
-
Effects of growth and measurement light intensities on temperature dependence of CO(2) assimilation rate in tobacco leaves.Plant Cell Environ. 2010 Mar;33(3):332-43. doi: 10.1111/j.1365-3040.2009.02067.x. Epub 2009 Nov 4. Plant Cell Environ. 2010. PMID: 19895395
-
Spatial variation in photosynthetic CO(2) carbon and oxygen isotope discrimination along leaves of the monocot triticale (Triticum × Secale) relates to mesophyll conductance and the Péclet effect.Plant Cell Environ. 2011 Sep;34(9):1548-62. doi: 10.1111/j.1365-3040.2011.02352.x. Epub 2011 Jun 28. Plant Cell Environ. 2011. PMID: 21707646
-
The temperature response of C(3) and C(4) photosynthesis.Plant Cell Environ. 2007 Sep;30(9):1086-106. doi: 10.1111/j.1365-3040.2007.01682.x. Plant Cell Environ. 2007. PMID: 17661749 Review.
-
Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field.J Exp Bot. 2009;60(8):2249-70. doi: 10.1093/jxb/erp036. Epub 2009 Apr 23. J Exp Bot. 2009. PMID: 19395391 Review.
Cited by
-
The qTSN4 Effect on Flag Leaf Size, Photosynthesis and Panicle Size, Benefits to Plant Grain Production in Rice, Depending on Light Availability.Front Plant Sci. 2016 May 10;7:623. doi: 10.3389/fpls.2016.00623. eCollection 2016. Front Plant Sci. 2016. PMID: 27242827 Free PMC article.
-
Time-series RNA-Seq transcriptome profiling reveals novel insights about cold acclimation and de-acclimation processes in an evergreen shrub of high altitude.Sci Rep. 2022 Sep 16;12(1):15553. doi: 10.1038/s41598-022-19834-w. Sci Rep. 2022. PMID: 36114408 Free PMC article.
-
The contribution of PIP2-type aquaporins to photosynthetic response to increased vapour pressure deficit.J Exp Bot. 2021 Jun 22;72(13):5066-5078. doi: 10.1093/jxb/erab187. J Exp Bot. 2021. PMID: 33928350 Free PMC article.
-
A microscale model for combined CO(2) diffusion and photosynthesis in leaves.PLoS One. 2012;7(11):e48376. doi: 10.1371/journal.pone.0048376. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144870 Free PMC article.
-
Optimum root zone temperature of photosynthesis and plant growth depends on air temperature in lettuce plants.Plant Mol Biol. 2022 Nov;110(4-5):385-395. doi: 10.1007/s11103-022-01249-w. Epub 2022 Feb 15. Plant Mol Biol. 2022. PMID: 35169910
References
-
- Badger MR. Photosynthetic oxygen exchange. Annu Rev Plant Physiol. 1985;36:27–53.
-
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001;24:253–259.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

