Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251
- PMID: 12477823
- PMCID: PMC140621
- DOI: 10.1128/jvi.77.1.179-190.2003
Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251
Abstract
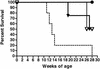
There is an urgent need for active immunization strategies that, if administered shortly after birth, could protect infants in developing countries from acquiring human immunodeficiency virus (HIV) infection through breast-feeding. Better knowledge of the immunogenic properties of vaccine candidates in infants and of the effect of maternal antibodies on vaccine efficacy will aid in the development of such a neonatal HIV vaccine. Simian immunodeficiency virus (SIV) infection of infant macaques is a useful animal model of pediatric HIV infection with which to address these questions. Groups of infant macaques were immunized at birth and 3 weeks of age with either modified vaccinia virus Ankara (MVA) expressing SIV Gag, Pol, and Env (MVA-SIVgpe) or live-attenuated SIVmac1A11. One MVA-SIVgpe-immunized group had maternally derived anti-SIV antibodies prior to immunization. Animals were challenged orally at 4 weeks of age with a genetically heterogeneous stock of virulent SIVmac251. Although all animals became infected, the immunized animals mounted better antiviral antibody responses, controlled virus levels more effectively, and had a longer disease-free survival than the unvaccinated infected monkeys. Maternal antibodies did not significantly reduce the efficacy of the MVA-SIVgpe vaccine. In conclusion, although the tested vaccines delayed the onset of AIDS, further studies are warranted to determine whether a vaccine that elicits stronger early immune responses at the time of virus exposure may be able to prevent viral infection or AIDS in infants.
Figures



Similar articles
-
Simian immunodeficiency virus (SIV) envelope quasispecies transmission and evolution in infant rhesus macaques after oral challenge with uncloned SIVmac251: increased diversity is associated with neutralizing antibodies and improved survival in previously immunized animals.Virol J. 2005 Feb 14;2:11. doi: 10.1186/1743-422X-2-11. Virol J. 2005. PMID: 15710048 Free PMC article.
-
Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV.Vaccine. 2010 Feb 10;28(6):1481-92. doi: 10.1016/j.vaccine.2009.11.061. Epub 2009 Dec 6. Vaccine. 2010. PMID: 19995539 Free PMC article.
-
Vaccine-Elicited Mucosal and Systemic Antibody Responses Are Associated with Reduced Simian Immunodeficiency Viremia in Infant Rhesus Macaques.J Virol. 2016 Jul 27;90(16):7285-7302. doi: 10.1128/JVI.00481-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252535 Free PMC article.
-
The rhesus macaque pediatric SIV infection model - a valuable tool in understanding infant HIV-1 pathogenesis and for designing pediatric HIV-1 prevention strategies.Curr HIV Res. 2009 Jan;7(1):2-11. doi: 10.2174/157016209787048528. Curr HIV Res. 2009. PMID: 19149549 Free PMC article. Review.
-
Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates.Adv Exp Med Biol. 1996;397:7-13. doi: 10.1007/978-1-4899-1382-1_2. Adv Exp Med Biol. 1996. PMID: 8718576 Free PMC article. Review.
Cited by
-
Viral sequence diversity: challenges for AIDS vaccine designs.Expert Rev Vaccines. 2008 Nov;7(9):1405-17. doi: 10.1586/14760584.7.9.1405. Expert Rev Vaccines. 2008. PMID: 18980542 Free PMC article. Review.
-
A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations.J Virol. 2004 Dec;78(24):14048-52. doi: 10.1128/JVI.78.24.14048-14052.2004. J Virol. 2004. PMID: 15564513 Free PMC article.
-
Simian immunodeficiency virus (SIV) envelope quasispecies transmission and evolution in infant rhesus macaques after oral challenge with uncloned SIVmac251: increased diversity is associated with neutralizing antibodies and improved survival in previously immunized animals.Virol J. 2005 Feb 14;2:11. doi: 10.1186/1743-422X-2-11. Virol J. 2005. PMID: 15710048 Free PMC article.
-
Different adjuvanted pediatric HIV envelope vaccines induced distinct plasma antibody responses despite similar B cell receptor repertoires in infant rhesus macaques.PLoS One. 2021 Dec 31;16(12):e0256885. doi: 10.1371/journal.pone.0256885. eCollection 2021. PLoS One. 2021. PMID: 34972105 Free PMC article.
-
More than the Infinite Monkey Theorem: NHP Models in the Development of a Pediatric HIV Cure.Curr HIV/AIDS Rep. 2024 Feb;21(1):11-29. doi: 10.1007/s11904-023-00686-6. Epub 2024 Jan 16. Curr HIV/AIDS Rep. 2024. PMID: 38227162 Free PMC article. Review.
References
-
- Abel, K., M. J. Alegria-Hartman, K. Zanotto, M. B. McChesney, M. L. Marthas, and C. J. Miller. 2001. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal rhesus macaques. Cytokine 16:191-204. - PubMed
-
- Bertolli, J., M. E. St. Louis, R. J. Simonds, P. Nieburg, M. Kamenga, C. Brown, M. Tarande, T. Quinn, and C. Y. Ou. 1996. Estimating the timing of mother-to-child transmission of human immunodeficiency virus in a breast-feeding population in Kinshasa, Zaire. J. Infect. Dis. 174:722-726. - PubMed
-
- Blanchard, T. J., A. Alcami, P. Andrea, and G. L. Smith. 1998. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 79:1159-1167. - PubMed
-
- Borkowsky, W., D. Wara, T. Fenton, J. McNamara, M. Kang, L. Mofenson, E. McFarland, C. Cunningham, A.-M. Duliege, D. Francis, Y. Bryson, S. Burchett, S. A. Spector, L. M. Frenkel, S. Starr, R. Van Dyke, E. Jiminez, et al. 2000. Lymphoproliferative responses to recombinant HIV-1 envelope antigens in neonates and infants receiving gp120 vaccines. J. Infect. Dis. 181:890-896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

