Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion
- PMID: 12473693
- PMCID: PMC2173392
- DOI: 10.1083/jcb.200206043
Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion
Abstract
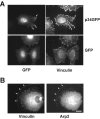
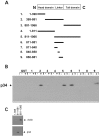
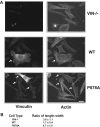
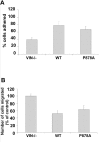
Cell migration involves many steps, including membrane protrusion and the development of new adhesions. Here we have investigated whether there is a link between actin polymerization and integrin engagement. In response to signals that trigger membrane protrusion, the actin-related protein (Arp)2/3 complex transiently binds to vinculin, an integrin-associated protein. The interaction is regulated, requiring phosphatidylinositol-4,5-bisphosphate and Rac1 activation, and is sufficient to recruit the Arp2/3 complex to new sites of integrin aggregation. Binding of the Arp2/3 complex to vinculin is direct and does not depend on the ability of vinculin to associate with actin. We have mapped the binding site for the Arp2/3 complex to the hinge region of vinculin, and a point mutation in this region selectively blocks binding to the Arp2/3 complex. Compared with WT vinculin, expression of this mutant in vinculin-null cells results in diminished lamellipodial protrusion and spreading on fibronectin. The recruitment of the Arp2/3 complex to vinculin may be one mechanism through which actin polymerization and membrane protrusion are coupled to integrin-mediated adhesion.
Figures



Similar articles
-
Lamellipodia protrusion: moving interactions of vinculin and Arp2/3.Curr Biol. 2003 Mar 18;13(6):R236-8. doi: 10.1016/s0960-9822(03)00160-x. Curr Biol. 2003. PMID: 12646151
-
Muscle costameric protein, Chisel/Smpx, associates with focal adhesion complexes and modulates cell spreading in vitro via a Rac1/p38 pathway.Exp Cell Res. 2005 Jul 15;307(2):367-80. doi: 10.1016/j.yexcr.2005.04.006. Exp Cell Res. 2005. PMID: 15893749
-
Adhesive F-actin waves: a novel integrin-mediated adhesion complex coupled to ventral actin polymerization.PLoS One. 2011;6(11):e26631. doi: 10.1371/journal.pone.0026631. Epub 2011 Nov 1. PLoS One. 2011. PMID: 22069459 Free PMC article.
-
Integrin-mediated cell adhesion: the cytoskeletal connection.Biochem Soc Symp. 1999;65:79-99. Biochem Soc Symp. 1999. PMID: 10320934 Review.
-
The web and the rock: cell adhesion and the ARP2/3 complex.Dev Cell. 2002 Dec;3(6):760-1. doi: 10.1016/s1534-5807(02)00374-x. Dev Cell. 2002. PMID: 12479800 Review.
Cited by
-
Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth.J Cell Biol. 2013 Jul 8;202(1):163-77. doi: 10.1083/jcb.201303129. J Cell Biol. 2013. PMID: 23836933 Free PMC article.
-
Mechanotransduction at focal adhesions: integrating cytoskeletal mechanics in migrating cells.J Cell Mol Med. 2013 Jun;17(6):704-12. doi: 10.1111/jcmm.12054. Epub 2013 Apr 4. J Cell Mol Med. 2013. PMID: 23551528 Free PMC article. Review.
-
Focal adhesion kinases in adhesion structures and disease.J Signal Transduct. 2012;2012:296450. doi: 10.1155/2012/296450. Epub 2012 Jul 19. J Signal Transduct. 2012. PMID: 22888421 Free PMC article.
-
At the Start of the Sarcomere: A Previously Unrecognized Role for Myosin Chaperones and Associated Proteins during Early Myofibrillogenesis.Biochem Res Int. 2012;2012:712315. doi: 10.1155/2012/712315. Epub 2012 Jan 30. Biochem Res Int. 2012. PMID: 22400118 Free PMC article.
-
Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension.J Cell Sci. 2012 Nov 1;125(Pt 21):5110-23. doi: 10.1242/jcs.108035. Epub 2012 Aug 16. J Cell Sci. 2012. PMID: 22899715 Free PMC article.
References
-
- Aspenstrom, P., U. Lindberg, and A. Hall. 1996. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr. Biol. 6:70–75. - PubMed
-
- Bagrodia, S., S.J. Taylor, K.A. Jordon, L. Van Aelst, and R.A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633–23636. - PubMed
-
- Bailly, M., I. Ichetovkin, W. Grant, N. Zebda, L.M. Machesky, J.E. Segall, and J. Condeelis. 2001. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr. Biol. 11:620–625. - PubMed
-
- Blanchoin, L., K.J. Amann, H.N. Higgs, J.B. Marchand, D.A. Kaiser, and T.D. Pollard. 2000. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 404:1007–1011. - PubMed
-
- Borisy, G.G., and T.M. Svitkina. 2000. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12:104–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

