Gibberellins are required for seed development and pollen tube growth in Arabidopsis
- PMID: 12468732
- PMCID: PMC151207
- DOI: 10.1105/tpc.003046
Gibberellins are required for seed development and pollen tube growth in Arabidopsis
Abstract
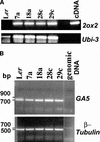
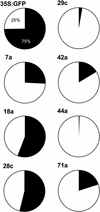
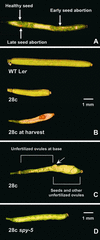
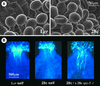
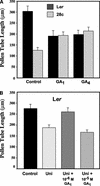
Gibberellins (GAs) are tetracyclic diterpenoids that are essential endogenous regulators of plant growth and development. GA levels within the plant are regulated by a homeostatic mechanism that includes changes in the expression of a family of GA-inactivating enzymes known as GA 2-oxidases. Ectopic expression of a pea GA 2-oxidase2 cDNA caused seed abortion in Arabidopsis, extending and confirming previous observations obtained with GA-deficient mutants of pea, suggesting that GAs have an essential role in seed development. A new physiological role for GAs in pollen tube growth in vivo also has been identified. The growth of pollen tubes carrying the 35S:2ox2 transgene was reduced relative to that of nontransgenic pollen, and this phenotype could be reversed partially by GA application in vitro or by combining with spy-5, a mutation that increases GA response. Treatment of wild-type pollen tubes with an inhibitor of GA biosynthesis in vitro also suggested that GAs are required for normal pollen tube growth. These results extend the known physiological roles of GAs in Arabidopsis development and suggest that GAs are required for normal pollen tube growth, a physiological role for GAs that has not been established previously.
Figures

Similar articles
-
The gar2 and rga alleles increase the growth of gibberellin-deficient pollen tubes in Arabidopsis.Plant Physiol. 2004 Feb;134(2):694-705. doi: 10.1104/pp.103.031666. Epub 2004 Feb 5. Plant Physiol. 2004. PMID: 14764903 Free PMC article.
-
Overexpression of a gibberellin inactivation gene alters seed development, KNOX gene expression, and plant development in Arabidopsis.Physiol Plant. 2010 Jan;138(1):74-90. doi: 10.1111/j.1399-3054.2009.01289.x. Epub 2009 Sep 8. Physiol Plant. 2010. PMID: 19825007
-
Pollination-, development-, and auxin-specific regulation of gibberellin 3beta-hydroxylase gene expression in pea fruit and seeds.Plant Physiol. 2003 Mar;131(3):1137-46. doi: 10.1104/pp.102.015974. Plant Physiol. 2003. PMID: 12644664 Free PMC article.
-
Regulation of gibberellin biosynthesis by light.Curr Opin Plant Biol. 1999 Oct;2(5):398-403. doi: 10.1016/s1369-5266(99)00012-6. Curr Opin Plant Biol. 1999. PMID: 10508758 Review.
-
Tall tales from sly dwarves: novel functions of gibberellins in plant development.Trends Plant Sci. 2005 Mar;10(3):123-9. doi: 10.1016/j.tplants.2005.01.007. Trends Plant Sci. 2005. PMID: 15749470 Review.
Cited by
-
Uncovering DELLA-Independent Gibberellin Responses by Characterizing New Tomato procera Mutants.Plant Cell. 2015 Jun;27(6):1579-94. doi: 10.1105/tpc.114.132795. Epub 2015 Jun 2. Plant Cell. 2015. PMID: 26036254 Free PMC article.
-
AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis.Plant Physiol. 2004 Nov;136(3):3660-9. doi: 10.1104/pp.104.047266. Epub 2004 Oct 29. Plant Physiol. 2004. PMID: 15516508 Free PMC article.
-
Ethrel-induced release of fresh seed dormancy causes remodelling of amylase activity, proteomics, phytohormone and fatty acid profile of groundnut (Arachis hypogaea L.).Physiol Mol Biol Plants. 2023 Jun;29(6):829-842. doi: 10.1007/s12298-023-01332-6. Epub 2023 Jul 19. Physiol Mol Biol Plants. 2023. PMID: 37520814 Free PMC article.
-
The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation.Plant Cell. 2011 May;23(5):1772-94. doi: 10.1105/tpc.111.085134. Epub 2011 May 13. Plant Cell. 2011. PMID: 21571950 Free PMC article.
-
Arabidopsis G-Protein β Subunit AGB1 Negatively Regulates DNA Binding of MYB62, a Suppressor in the Gibberellin Pathway.Int J Mol Sci. 2021 Jul 31;22(15):8270. doi: 10.3390/ijms22158270. Int J Mol Sci. 2021. PMID: 34361039 Free PMC article.
References
-
- Bagnall, D.J. (1992). Control of flowering in Arabidopsis thaliana by light, vernalization, and gibberellins. Aust. J. Plant Physiol. 19, 401–409.
-
- Barendse, G.W.M., Rodrigues-Pereira, A.J., Berkers, P.A., Driessen, F.M., van Eyden-Emons, A., and Linskens, H.F. (1970). Growth hormones in pollen, styles and ovaries of Petunia hybrida and Lilium species. Acta Bot. Neerl. 19, 175–186.
-
- Bhandal, I.S., and Malik, C.P. (1979). Effect of gibberellic acid, (2-chloroethyl)phosphoric acid, actinomycin-D and cycloheximide on the activity and leaching of some hydrolases in pollen suspension cultures of Crotalaria juncea. Physiol. Plant. 45, 297–300.
-
- Chandler, P.M. (1999). Gibberellins and the regulation of growth in barley. In Abstracts ComBio 1999: Sym 01-01. Canberra, Australia.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

