Translesion replication of benzo[a]pyrene and benzo[c]phenanthrene diol epoxide adducts of deoxyadenosine and deoxyguanosine by human DNA polymerase iota
- PMID: 12466554
- PMCID: PMC137958
- DOI: 10.1093/nar/gkf643
Translesion replication of benzo[a]pyrene and benzo[c]phenanthrene diol epoxide adducts of deoxyadenosine and deoxyguanosine by human DNA polymerase iota
Abstract
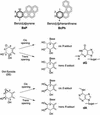
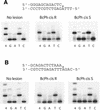
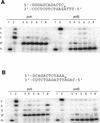
Human DNA polymerase iota (poliota) is a Y-family polymerase whose cellular function is presently unknown. Here, we report on the ability of poliota to bypass various stereoisomers of benzo[a]pyrene (BaP) diol epoxide (DE) and benzo[c]phenanthrene (BcPh) DE adducts at deoxyadenosine (dA) or deoxyguanosine (dG) bases in four different template sequence contexts in vitro. We find that the BaP DE dG adducts pose a strong block to poliota-dependent replication and result in a high frequency of base misincorporations. In contrast, misincorporations opposite BaP DE and BcPh DE dA adducts generally occurred with a frequency ranging between 2 x 10(-3) and 6 x 10(-4). Although dTMP was inserted efficiently opposite all dA adducts, further extension was relatively poor, with one exception (a cis opened adduct derived from BcPh DE) where up to 58% extension past the lesion was observed. Interestingly, another human Y-family polymerase, polkappa, was able to extend dTMP inserted opposite a BaP DE dA adduct. We suggest that poliota might therefore participate in the error-free bypass of DE-adducted dA in vivo by predominantly incorporating dTMP opposite the damaged base. In many cases, elongation would, however, require the participation of another polymerase more specialized in extension, such as polkappa.
Figures



Similar articles
-
Nucleotide incorporation by human DNA polymerase gamma opposite benzo[a]pyrene and benzo[c]phenanthrene diol epoxide adducts of deoxyguanosine and deoxyadenosine.Nucleic Acids Res. 2004 Jan 16;32(1):397-405. doi: 10.1093/nar/gkh213. Print 2004. Nucleic Acids Res. 2004. PMID: 14729924 Free PMC article.
-
Preferential misincorporation of purine nucleotides by human DNA polymerase eta opposite benzo[a]pyrene 7,8-diol 9,10-epoxide deoxyguanosine adducts.J Biol Chem. 2002 Apr 5;277(14):11765-71. doi: 10.1074/jbc.M112139200. Epub 2002 Jan 30. J Biol Chem. 2002. PMID: 11821420
-
Inhibition of Werner syndrome helicase activity by benzo[a]pyrene diol epoxide adducts can be overcome by replication protein A.J Biol Chem. 2006 Mar 3;281(9):6000-9. doi: 10.1074/jbc.M510122200. Epub 2005 Dec 27. J Biol Chem. 2006. PMID: 16380375
-
poliota-dependent lesion bypass in vitro.Mutat Res. 2002 Dec 29;510(1-2):9-22. doi: 10.1016/s0027-5107(02)00248-8. Mutat Res. 2002. PMID: 12459439 Review.
-
A rescue act: Translesion DNA synthesis past N(2) -deoxyguanosine adducts.IUBMB Life. 2015 Jul;67(7):564-74. doi: 10.1002/iub.1403. Epub 2015 Jul 14. IUBMB Life. 2015. PMID: 26173005 Review.
Cited by
-
Kinetic and Structural Impact of Metal Ions and Genetic Variations on Human DNA Polymerase ι.J Biol Chem. 2016 Sep 30;291(40):21063-21073. doi: 10.1074/jbc.M116.748285. Epub 2016 Aug 23. J Biol Chem. 2016. PMID: 27555320 Free PMC article.
-
Translesion Synthesis of 2'-Deoxyguanosine Lesions by Eukaryotic DNA Polymerases.Chem Res Toxicol. 2017 Jan 17;30(1):61-72. doi: 10.1021/acs.chemrestox.6b00285. Epub 2016 Nov 1. Chem Res Toxicol. 2017. PMID: 27760288 Free PMC article.
-
Conformational searches elucidate effects of stereochemistry on structures of deoxyadenosine covalently bound to tumorigenic metabolites of benzo[C] phenanthrene.Front Biosci. 2004 Sep 1;9:2807-18. doi: 10.2741/1438. Front Biosci. 2004. PMID: 15353316 Free PMC article.
-
Influence of local sequence context on damaged base conformation in human DNA polymerase iota: molecular dynamics studies of nucleotide incorporation opposite a benzo[a]pyrene-derived adenine lesion.Nucleic Acids Res. 2009 Nov;37(21):7095-109. doi: 10.1093/nar/gkp745. Nucleic Acids Res. 2009. PMID: 19767609 Free PMC article.
-
Translesion synthesis of 6-nitrochrysene-derived 2'-deoxyadenosine adduct in human cells.DNA Repair (Amst). 2020 Nov;95:102935. doi: 10.1016/j.dnarep.2020.102935. Epub 2020 Jul 18. DNA Repair (Amst). 2020. PMID: 32721818 Free PMC article.
References
-
- Thakker D.R., Yagi,H., Levin,W., Wood,A.W., Conney,A.H. and Jerina,D.M. (1985) Polycyclic aromatic hydrocarbons: metabolic activation to ultimate carcinogenesis. In Anders,M.W. (ed.), Bioactivation of Foreign Compounds. Academic Press, New York, NY, pp. 177–242.
-
- Jerina D.M., Sayer,J.M., Agarwal,S.K., Yagi,H., Levin,W., Wood,A.W., Conney,A.H., Pruess-Schartz,D., Baird,W.M., Pigott,M.A. and Dipple,A. (1986) Reactivity and tumorigenicity of bay-region diol epoxides derived from polycyclic aromatic hydrocarbons. In Kocsis,J.J., Jollow,D.J., Witmer,C.M., Nelson,J.O. and Snyder,R. (eds), Biological Reactive Intermediates. Plenum Press, New York, NY, Vol. 3. pp. 11–30. - PubMed
-
- Jerina D.M., Yagi,H., Thakker,D.R., Sayer,J.M., van Bladeren,P.J., Lehr,R.E., Whalen,D.L., Levin,W., Chang,R.L., Wood,A.W. and Conney,A.H. (1984) Identification of ultimate carcinogenic metabolites of the polycyclic aromatic hydrocarbons: bay-region (R,S)-diol-(S,R)-epoxides. In Caldwell,J. and Paulson,G.D. (eds), Foreign Compound Metabolism. Taylor & Francis, London, UK, pp. 257–266.
-
- Jerina D.M., Chadha,A., Cheh,A.M., Schurdak,M.E., Wood,A.W. and Sayer,J.M. (1991) Covalent bonding of bay-region diol epoxides to nucleic acids. In Witmer,C.M., Snyder,R., Jollow,D.J., Kalf,G.F., Kocsis,J.J. and Sipes,I.G. (eds), Biological Reactive Intermediates IV. Molecular and Cellular Effects and Their Impact on Human Health. Plenum Press, New York, NY, pp. 533–553. - PubMed
-
- Cheng S.C., Hilton,B.D., Roman,J.M. and Dipple,A. (1989) DNA adducts from carcinogenic and noncarcinogenic enantiomers of benzo[a]pyrene dihydrodiol epoxide. Chem. Res. Toxicol., 2, 334–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

