Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo
- PMID: 12454288
- PMCID: PMC138567
- DOI: 10.1073/pnas.252649399
Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo
Abstract
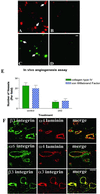
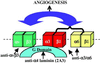
The alpha4 laminin subunit is a component of the basement membrane of blood vessels where it codistributes with the integrins alphavbeta3, alpha3beta1, and alpha6beta1. An antibody against the G domain (residues 919-1207; G(919-1207)) of the alpha4 laminin subunit inhibits angiogenesis in a mouse-human chimeric model, indicating the functional importance of this domain. Additional support for the latter derives from the ability of recombinant G(919-1207) to support endothelial cell adhesion. In particular, endothelial cell adhesion to G(919-1207) is half-maximal at 1.4 nM, whereas residues 919-1018 and 1016-1207 of the G domain are poor cellular ligands. Function blocking antibodies against integrins alphavbeta3 and beta1 and a combination of antibodies against alpha3 and alpha6 integrin subunits inhibit endothelial cell attachment to G(919-1207). Moreover, both alphavbeta3 and alpha3beta1 integrin bind with high affinity to G(919-1207). Together, our studies demonstrate that the G domain of laminin alpha4 chain is a specific, high affinity ligand for the alphavbeta3 and alpha3beta1 integrin heterodimers and that these integrins, together with alpha6beta1, function cooperatively to mediate endothelial cell-alpha4 laminin interaction and hence blood vessel development. We propose a model based on these data that reconcile apparent discrepancies in the recent literature with regard to the role of the alphavbeta3 integrin in angiogenesis.
Figures
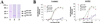
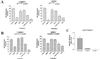

Similar articles
-
Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins.J Biol Chem. 2001 May 18;276(20):17550-8. doi: 10.1074/jbc.M010155200. Epub 2001 Feb 13. J Biol Chem. 2001. PMID: 11278628
-
Identification of an active site on the laminin alpha4 chain globular domain that binds to alphavbeta3 integrin and promotes angiogenesis.Biochem Biophys Res Commun. 2006 Aug 18;347(1):248-53. doi: 10.1016/j.bbrc.2006.06.069. Epub 2006 Jun 21. Biochem Biophys Res Commun. 2006. PMID: 16824487
-
Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment.Clin Exp Metastasis. 2005;22(5):391-402. doi: 10.1007/s10585-005-1262-y. Clin Exp Metastasis. 2005. PMID: 16283482
-
Novel integrin antagonists derived from thrombospondins.Curr Pharm Des. 2005;11(7):849-66. doi: 10.2174/1381612053381792. Curr Pharm Des. 2005. PMID: 15777239 Review.
-
Physiological and pathological roles of alpha3beta1 integrin.J Membr Biol. 2004 Aug 1;200(3):115-32. doi: 10.1007/s00232-004-0696-5. J Membr Biol. 2004. PMID: 15625821 Review.
Cited by
-
Intrauterine growth restriction decreases NF-κB signaling in fetal pulmonary artery endothelial cells of fetal sheep.Am J Physiol Lung Cell Mol Physiol. 2018 Sep 1;315(3):L348-L359. doi: 10.1152/ajplung.00052.2018. Epub 2018 May 3. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29722560 Free PMC article.
-
Integrin cross-talk in endothelial cells is regulated by protein kinase A and protein phosphatase 1.J Biol Chem. 2008 Nov 14;283(46):31849-60. doi: 10.1074/jbc.M801345200. Epub 2008 Sep 19. J Biol Chem. 2008. PMID: 18806263 Free PMC article.
-
NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin.Mol Biol Cell. 2004 Aug;15(8):3580-90. doi: 10.1091/mbc.e04-03-0236. Epub 2004 Jun 4. Mol Biol Cell. 2004. PMID: 15181153 Free PMC article.
-
Lung-specific loss of the laminin α3 subunit confers resistance to mechanical injury.J Cell Sci. 2011 Sep 1;124(Pt 17):2927-37. doi: 10.1242/jcs.080911. J Cell Sci. 2011. PMID: 21878500 Free PMC article.
-
RGD-Binding Integrins in Prostate Cancer: Expression Patterns and Therapeutic Prospects against Bone Metastasis.Cancers (Basel). 2012 Oct 26;4(4):1106-45. doi: 10.3390/cancers4041106. Cancers (Basel). 2012. PMID: 24213501 Free PMC article.
References
-
- Timpl R. & Brown, J. C. (1994) Matrix Biol. 14, 275-281. - PubMed
-
- Tunggal P., Smyth, N., Paulsson, M. & Ott, M.-C. (2000) Microsc. Res. Tech. 51, 214-227. - PubMed
-
- Smirnov S., McDearmon, E., Shaohua, L., Ervasti, J., Tryggvason, K. & Yurchenco, P. D. (2002) J. Biol. Chem. 277, 18928-18937. - PubMed
-
- Patton B. L. (2000) Microsc. Res. Tech. 51, 247-261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

