Nitric oxide preferentially induces type 1 T cell differentiation by selectively up-regulating IL-12 receptor beta 2 expression via cGMP
- PMID: 12451176
- PMCID: PMC138586
- DOI: 10.1073/pnas.252464599
Nitric oxide preferentially induces type 1 T cell differentiation by selectively up-regulating IL-12 receptor beta 2 expression via cGMP
Abstract
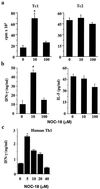
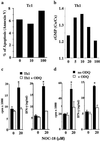
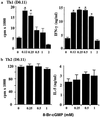
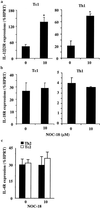
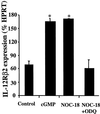
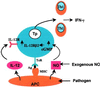
Nitric oxide plays an important role in immune regulation. We have shown that although high concentrations of NO generally were immune-suppressive, low concentrations of NO selectively enhanced the differentiation of T helper (Th)1 cells but not Th2 cells. This finding provided an explanation for the crucial role of NO in defense against intracellular pathogens. However, the mechanism for the selective induction of Th1 cells was unknown. We report here that at low concentrations, NO activates soluble guanylyl cyclase, leading to the up-regulation of cGMP, which selectively induces the expression of IL-12 receptor beta2 but has no effect on IL-4 receptor. Because IL-12 and IL-4 are the key cytokines for induction of Th1 and Th2 cells, respectively, these results, therefore, provide the mechanism for the selective action of NO on T cell subset differentiation. Furthermore, this selectivity also applies to CD8+ cytotoxic and human T cells and, thus, demonstrates the general implication of this observation in immune regulation. Our results also provide an example of the regulation of cytokine receptor expression by NO. The selectivity of such action via cGMP suggests that it is amenable to therapeutic intervention.
Figures
Similar articles
-
Agonist-specific compartmentation of cGMP action in myometrium.J Pharmacol Exp Ther. 2010 Oct;335(1):256-63. doi: 10.1124/jpet.110.171934. Epub 2010 Jul 22. J Pharmacol Exp Ther. 2010. PMID: 20651027 Free PMC article.
-
Nitric oxide reduces T lymphocyte adhesion to human brain microvessel endothelial cells via a cGMP-dependent pathway.Eur J Pharmacol. 2005 May 9;514(2-3):91-8. doi: 10.1016/j.ejphar.2005.03.025. Eur J Pharmacol. 2005. PMID: 15910796
-
Nitric oxide attenuates endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 in vascular smooth muscle cells by a cGMP-dependent pathway.Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2072-9. doi: 10.1152/ajpheart.01097.2006. Epub 2007 Jul 20. Am J Physiol Heart Circ Physiol. 2007. PMID: 17644565
-
[Nitric oxide. Potentiation of NO-dependent activation of soluble guanylate cyclase--(patho)physiological and pharmacotherapeutical significance].Biomed Khim. 2007 Jul-Aug;53(4):385-99. Biomed Khim. 2007. PMID: 18035720 Review. Russian.
-
Role of nitric oxide in the regulation of T cell functions.Ann Rheum Dis. 2006 Nov;65 Suppl 3(Suppl 3):iii37-40. doi: 10.1136/ard.2006.058446. Ann Rheum Dis. 2006. PMID: 17038470 Free PMC article. Review.
Cited by
-
Comparative studies on the roles of mediator molecules in expression of the suppressor activity of Mycobacterium avium complex-induced immunosuppressive macrophages against T cell and B cell mitogenic responses.Clin Exp Immunol. 2006 Mar;143(3):560-71. doi: 10.1111/j.1365-2249.2006.03016.x. Clin Exp Immunol. 2006. PMID: 16487256 Free PMC article.
-
Laminin signals initiate the reciprocal loop that informs breast-specific gene expression and homeostasis by activating NO, p53 and microRNAs.Elife. 2018 Mar 21;7:e26148. doi: 10.7554/eLife.26148. Elife. 2018. PMID: 29560860 Free PMC article.
-
Saying no to SARS-CoV-2: the potential of nitric oxide in the treatment of COVID-19 pneumonia.Med Gas Res. 2024 Apr-Jun;14(2):39-47. doi: 10.4103/2045-9912.385414. Med Gas Res. 2024. PMID: 37929506 Free PMC article. Review.
-
Poly(cyclodextrin)-Polydrug Nanocomplexes as Synthetic Oncolytic Virus for Locoregional Melanoma Chemoimmunotherapy.Adv Funct Mater. 2020 Apr 20;30(16):1908788. doi: 10.1002/adfm.201908788. Epub 2020 Feb 24. Adv Funct Mater. 2020. PMID: 33071710 Free PMC article.
-
Inhibition of HSV-1 by chemoattracted neutrophils: supernatants of corneal epithelial cells (HCE) and macrophages (THP-1) treated with virus components chemoattract neutrophils (PMN), and supernatants of PMN treated with these conditioned media inhibit viral growth.Arch Virol. 2012 Jul;157(7):1377-81. doi: 10.1007/s00705-012-1306-y. Epub 2012 Apr 12. Arch Virol. 2012. PMID: 22527863 Free PMC article.
References
-
- Moncada S., Palmer, R. M. & Higgs, E. A. (1991) Pharmacol. Rev. 43, 109-142. - PubMed
-
- Nathan C. & Xie, Q. W. (1994) Cell 78, 915-918. - PubMed
-
- Vladutiu A. O. (1995) Clin. Immunol. Immunopathol. 76, 1-11. - PubMed
-
- Liew F. Y. (1995) Curr. Opin. Immunol. 7, 396-399. - PubMed
-
- Kolb H. & Kolb-Bachofen, V. (1998) Immunol. Today 19, 556-561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

