Histone deacetylase activity is necessary for oligodendrocyte lineage progression
- PMID: 12451133
- PMCID: PMC6758756
- DOI: 10.1523/JNEUROSCI.22-23-10333.2002
Histone deacetylase activity is necessary for oligodendrocyte lineage progression
Abstract
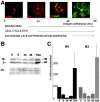
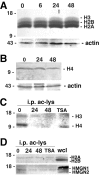
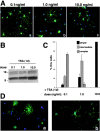
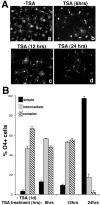
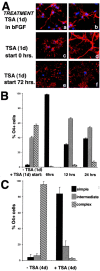
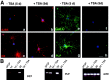
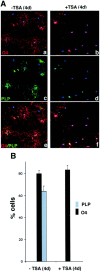
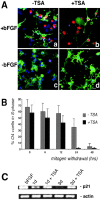
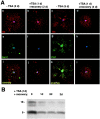
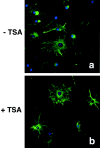
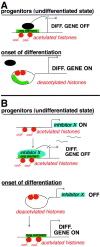
Gene expression can be modulated by chromatin changes induced by histone acetylation and deacetylation. Acetylation of histone lysine residues by acetyltransferases is associated with transcriptionally active chromatin, whereas the removal of acetyl groups by histone deacetylases (HDACs) correlates with repressed chromatin. Recent evidence has shown that histone deacetylation is responsible for restricting neuronal gene expression, whereas histone acetylation is necessary for astrocytic differentiation We now asked whether histone acetylation or deacetylation was necessary for oligodendrocyte differentiation. Neonatal rat cortical progenitors were kept proliferating and undifferentiated in the presence of mitogens and induced to stop proliferating and differentiate into oligodendrocytes by mitogen removal. Histone deacetylation was observed during the temporal window between exit from the cell cycle and onset of differentiation, which was characterized by acquisition of branched morphology and myelin gene expression. Blocking HDAC activity during this critical window using the inhibitor trichostatin A (TSA) prevented the progression of progenitors into mature oligodendrocytes. TSA-treated progenitors were able to exit from the cell cycle but did not progress to oligodendrocytes. Their development was arrested at the progenitor stage, characterized by simple morphology and lack of myelin gene expression. The effect of TSA on progenitor differentiation was lineage specific, because TSA did not affect the ability of these cells to differentiate into type II astrocytes when cultured in the presence of serum. From these data, we conclude that histone deacetylation is a necessary component of the oligodendrocyte differentiation program.
Figures
Similar articles
-
Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain.J Cell Biol. 2005 May 23;169(4):577-89. doi: 10.1083/jcb.200412101. Epub 2005 May 16. J Cell Biol. 2005. PMID: 15897262 Free PMC article.
-
Effects of histone deacetylation inhibition on neuronal differentiation of embryonic mouse neural stem cells.Neuroscience. 2006 Dec 28;143(4):939-51. doi: 10.1016/j.neuroscience.2006.08.082. Epub 2006 Nov 3. Neuroscience. 2006. PMID: 17084985
-
Inhibition of histone deacetylases by Trichostatin A leads to a HoxB4-independent increase of hematopoietic progenitor/stem cell frequencies as a result of selective survival.Cytotherapy. 2010 Nov;12(7):899-908. doi: 10.3109/14653240903580254. Cytotherapy. 2010. PMID: 20210674
-
Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: toxicological point of view.Curr Med Chem. 2004 Jun;11(12):1629-43. doi: 10.2174/0929867043365099. Curr Med Chem. 2004. PMID: 15180568 Review.
-
Zn(II)-dependent histone deacetylase inhibitors: suberoylanilide hydroxamic acid and trichostatin A.Int J Biochem Cell Biol. 2009 Apr;41(4):736-9. doi: 10.1016/j.biocel.2008.05.026. Epub 2008 Aug 3. Int J Biochem Cell Biol. 2009. PMID: 18725319 Review.
Cited by
-
Acetate supplementation as a means of inducing glioblastoma stem-like cell growth arrest.J Cell Physiol. 2015 Aug;230(8):1929-43. doi: 10.1002/jcp.24927. J Cell Physiol. 2015. PMID: 25573156 Free PMC article.
-
Coordinated Regulation of Myelination by Growth Factor and Amino-acid Signaling Pathways.Neurosci Bull. 2023 Mar;39(3):453-465. doi: 10.1007/s12264-022-00967-x. Epub 2022 Nov 9. Neurosci Bull. 2023. PMID: 36352321 Free PMC article. Review.
-
Histone deacetylase expression patterns in developing murine optic nerve.BMC Dev Biol. 2014 Jul 9;14:30. doi: 10.1186/1471-213X-14-30. BMC Dev Biol. 2014. PMID: 25011550 Free PMC article.
-
Exercise in Adolescence Enhances Callosal White Matter Refinement in the Female Brain in a Rat Model of Fetal Alcohol Spectrum Disorders.Cells. 2023 Mar 23;12(7):975. doi: 10.3390/cells12070975. Cells. 2023. PMID: 37048047 Free PMC article.
-
Glia Disease and Repair-Remyelination.Cold Spring Harb Perspect Biol. 2015 May 18;7(7):a020594. doi: 10.1101/cshperspect.a020594. Cold Spring Harb Perspect Biol. 2015. PMID: 25986556 Free PMC article. Review.
References
-
- Ashraf SI, Ip YT. Transcriptional control: repression by local chromatin modification. Curr Biol. 1998;8:R683–R686. - PubMed
-
- Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. - PubMed
-
- Bansal R, Pfeiffer SE. FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res. 1997;50:215–228. - PubMed
-
- Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989;24:548–557. - PubMed
-
- Bansal R, Kumar M, Murray K, Morrison R, Pfeiffer SE. Regulation of FGF receptors in the oligodendrocyte lineage. Mol Cell Neurosci. 1996;7:263–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
