Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus
- PMID: 12451119
- PMCID: PMC6758770
- DOI: 10.1523/JNEUROSCI.22-23-10182.2002
Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus
Abstract
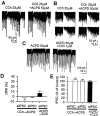
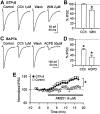
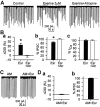
Endogenous cannabinoids (endocannabinoids) are endogenous compounds that resemble the active ingredient of marijuana and activate the cannabinoid receptor in the brain. They mediate retrograde signaling from principal cells to both inhibitory ["depolarization-induced suppression of inhibition" (DSI)] and excitatory ("depolarization-induced suppression of excitation") afferent fibers. Transient endocannabinoid release is triggered by voltage-dependent Ca(2+) influx and is upregulated by group I metabotropic glutamate receptor activation. Here we show that muscarinic acetylcholine receptor (mAChR) activation also enhances transient endocannabinoid release (DSI) and induces persistent release. Inhibitory synapses in the rat hippocampal CA1 region of acute slices were studied using whole-cell patch-clamp techniques. We found that low concentrations (0.2-0.5 microm) of carbachol (CCh) enhanced DSI without affecting basal evoked IPSCs (eIPSCs) by activating mAChRs on postsynaptic cells. Higher concentrations of CCh (> or =1 microm) enhanced DSI and also persistently depressed basal eIPSCs, mainly by releasing endocannabinoids. Persistent CCh-induced endocannabinoid release did not require an increase in [Ca2+]i but was dependent on G-proteins. Although they were independent at the receptor level, muscarinic and glutamatergic mechanisms of endocannabinoid release shared intracellular machinery. Replication of the effects of CCh by blocking acetylcholinesterase with eserine suggests that mAChR-mediated endocannabinoid release is physiologically relevant. This study reveals a new role of the muscarinic cholinergic system in mammalian brain.
Figures
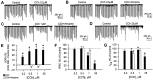
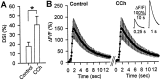
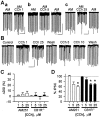
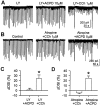
Similar articles
-
Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus.J Neurosci. 2001 Dec 15;21(24):RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. J Neurosci. 2001. PMID: 11734603 Free PMC article.
-
Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses.J Neurosci. 2002 May 15;22(10):3864-72. doi: 10.1523/JNEUROSCI.22-10-03864.2002. J Neurosci. 2002. PMID: 12019305 Free PMC article.
-
Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus.Eur J Neurosci. 2003 Jul;18(1):109-16. doi: 10.1046/j.1460-9568.2003.02732.x. Eur J Neurosci. 2003. PMID: 12859343
-
Cellular signaling mechanisms for muscarinic acetylcholine receptors.Recept Channels. 2003;9(4):241-60. Recept Channels. 2003. PMID: 12893537 Review.
-
Muscarinic cholinergic receptors modulate inhibitory synaptic rhythms in hippocampus and neocortex.Front Synaptic Neurosci. 2014 Sep 5;6:18. doi: 10.3389/fnsyn.2014.00018. eCollection 2014. Front Synaptic Neurosci. 2014. PMID: 25249974 Free PMC article. Review.
Cited by
-
Endocannabinoid 2-Arachidonoylglycerol Synthesis and Metabolism at Neuronal Nuclear Matrix Fractions Derived from Adult Rat Brain Cortex.Int J Mol Sci. 2023 Feb 5;24(4):3165. doi: 10.3390/ijms24043165. Int J Mol Sci. 2023. PMID: 36834575 Free PMC article.
-
Search for an endogenous cannabinoid-mediated effect in the sympathetic nervous system.Naunyn Schmiedebergs Arch Pharmacol. 2005 Jan;371(1):9-17. doi: 10.1007/s00210-004-1003-9. Epub 2005 Jan 20. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15660243
-
Convergent Metabotropic Signaling Pathways Inhibit SK Channels to Promote Synaptic Plasticity in the Hippocampus.J Neurosci. 2018 Oct 24;38(43):9252-9262. doi: 10.1523/JNEUROSCI.1160-18.2018. Epub 2018 Sep 21. J Neurosci. 2018. PMID: 30242046 Free PMC article.
-
Optogenetic identification of an intrinsic cholinergically driven inhibitory oscillator sensitive to cannabinoids and opioids in hippocampal CA1.J Physiol. 2014 Jan 1;592(1):103-23. doi: 10.1113/jphysiol.2013.257428. Epub 2013 Nov 4. J Physiol. 2014. PMID: 24190932 Free PMC article.
-
Cannabidiol Inhibits Endocannabinoid Signaling in Autaptic Hippocampal Neurons.Mol Pharmacol. 2018 Jul;94(1):743-748. doi: 10.1124/mol.118.111864. Epub 2018 Apr 18. Mol Pharmacol. 2018. PMID: 29669714 Free PMC article.
References
-
- Alger BE, Pitler TA. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18:333–340. - PubMed
-
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. - PubMed
-
- Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
