The Epstein-Barr virus immediate-early protein BZLF1 induces expression of E2F-1 and other proteins involved in cell cycle progression in primary keratinocytes and gastric carcinoma cells
- PMID: 12438580
- PMCID: PMC136734
- DOI: 10.1128/jvi.76.24.12543-12552.2002
The Epstein-Barr virus immediate-early protein BZLF1 induces expression of E2F-1 and other proteins involved in cell cycle progression in primary keratinocytes and gastric carcinoma cells
Abstract
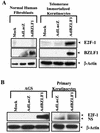
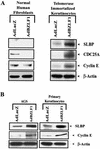
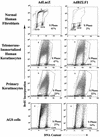
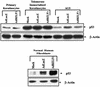
The Epstein-Barr virus (EBV) immediate-early protein BZLF1 mediates the switch between the latent and lytic forms of EBV infection and has been previously shown to induce a G(1)/S block in cell cycle progression in some cell types. To examine the effect of BZLF1 on cellular gene expression, we performed microarray analysis on telomerase-immortalized human keratinocytes that were mock infected or infected with a control adenovirus vector (AdLacZ) or a vector expressing the EBV BZLF1 protein (AdBZLF1). Cellular genes activated by BZLF1 expression included E2F-1, cyclin E, Cdc25A, and a number of other genes involved in cell cycle progression. Immunoblot analysis confirmed that BZLF1 induced expression of E2F-1, cyclin E, Cdc25A, and stem loop binding protein (a protein known to be primarily expressed during S phase) in telomerase-immortalized keratinocytes. Similarly, BZLF1 increased expression of E2F-1, cyclin E, and stem loop binding protein (SLBP) in primary tonsil keratinocytes. In contrast, BZLF1 did not induce E2F-1 expression in normal human fibroblasts. Cell cycle analysis revealed that while BZLF1 dramatically blocked G(1)/S progression in normal human fibroblasts, it did not significantly affect cell cycle progression in primary human tonsil keratinocytes. Furthermore, in EBV-infected gastric carcinoma cells, the BZLF1-positive cells had an increased number of cells in S phase compared to the BZLF1-negative cells. Thus, in certain cell types (but not others), BZLF1 enhances expression of cellular proteins associated with cell cycle progression, which suggests that an S-phase-like environment may be advantageous for efficient lytic EBV replication in some cell types.
Figures


Similar articles
-
Reactivation of lytic replication from B cells latently infected with Epstein-Barr virus occurs with high S-phase cyclin-dependent kinase activity while inhibiting cellular DNA replication.J Virol. 2003 Jan;77(2):851-61. doi: 10.1128/jvi.77.2.851-861.2003. J Virol. 2003. PMID: 12502801 Free PMC article.
-
The Epstein-Barr virus protein BRLF1 activates S phase entry through E2F1 induction.J Virol. 1999 Aug;73(8):6540-50. doi: 10.1128/JVI.73.8.6540-6550.1999. J Virol. 1999. PMID: 10400750 Free PMC article.
-
The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms.J Virol. 2002 Dec;76(24):12503-12. doi: 10.1128/jvi.76.24.12503-12512.2002. J Virol. 2002. PMID: 12438576 Free PMC article.
-
Regulation of the G1/S transition phase in mesangial cells by E2F1.Kidney Int. 1999 Oct;56(4):1238-41. doi: 10.1046/j.1523-1755.1999.00705.x. Kidney Int. 1999. PMID: 10504464 Review.
-
Extra-telomeric functions of telomerase in the pathogenesis of Epstein-Barr virus-driven B-cell malignancies and potential therapeutic implications.Infect Agent Cancer. 2018 Apr 10;13:14. doi: 10.1186/s13027-018-0186-5. eCollection 2018. Infect Agent Cancer. 2018. PMID: 29643934 Free PMC article. Review.
Cited by
-
Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa.Dig Dis Sci. 2012 Jul;57(7):1887-98. doi: 10.1007/s10620-012-2116-5. Epub 2012 Mar 13. Dig Dis Sci. 2012. PMID: 22410851 Free PMC article.
-
Epstein-Barr virus infection in ex vivo tonsil epithelial cell cultures of asymptomatic carriers.J Virol. 2004 Nov;78(22):12613-24. doi: 10.1128/JVI.78.22.12613-12624.2004. J Virol. 2004. PMID: 15507648 Free PMC article.
-
The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1.J Virol. 2004 May;78(10):4983-92. doi: 10.1128/jvi.78.10.4983-4992.2004. J Virol. 2004. PMID: 15113878 Free PMC article.
-
The zipper region of Epstein-Barr virus bZIP transcription factor Zta is necessary but not sufficient to direct DNA binding.J Virol. 2003 Jul;77(14):8173-7. doi: 10.1128/jvi.77.14.8173-8177.2003. J Virol. 2003. PMID: 12829857 Free PMC article.
-
The Epstein-Barr virus replication protein BBLF2/3 provides an origin-tethering function through interaction with the zinc finger DNA binding protein ZBRK1 and the KAP-1 corepressor.J Virol. 2005 Jan;79(1):245-56. doi: 10.1128/JVI.79.1.245-256.2005. J Virol. 2005. PMID: 15596820 Free PMC article.
References
-
- Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. - PMC - PubMed
-
- Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. - PubMed
-
- Bartek, J., and J. Lukas. 2001. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 490:117-122. - PubMed
-
- Boldogh, I., S. AbuBakar, and T. Albrecht. 1990. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science 247:561-564. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

