Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells
- PMID: 12432102
- PMCID: PMC137737
- DOI: 10.1073/pnas.202604299
Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells
Abstract
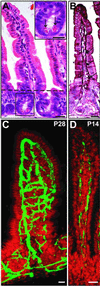
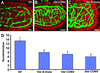
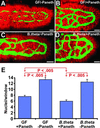
The adult mouse intestine contains an intricate vascular network. The factors that control development of this network are poorly understood. Quantitative three-dimensional imaging studies revealed that a plexus of branched interconnected vessels developed in small intestinal villi during the period of postnatal development that coincides with assembly of a complex society of indigenous gut microorganisms (microbiota). To investigate the impact of this environmental transition on vascular development, we compared the capillary networks of germ-free mice with those of ex-germ-free animals colonized during or after completion of postnatal gut development. Adult germ-free mice had arrested capillary network formation. The developmental program can be restarted and completed within 10 days after colonization with a complete microbiota harvested from conventionally raised mice, or with Bacteroides thetaiotaomicron, a prominent inhabitant of the normal mouse/human gut. Paneth cells in the intestinal epithelium secrete antibacterial peptides that affect luminal microbial ecology. Comparisons of germ-free and B. thetaiotaomicron-colonized transgenic mice lacking Paneth cells established that microbial regulation of angiogenesis depends on this lineage. These findings reveal a previously unappreciated mechanism of postnatal animal development, where microbes colonizing a mucosal surface are assigned responsibility for regulating elaboration of the underlying microvasculature by signaling through a bacteria-sensing epithelial cell.
Figures
Comment in
-
Commensal bacteria make a difference.Trends Microbiol. 2003 Apr;11(4):148-50. doi: 10.1016/s0966-842x(03)00038-6. Trends Microbiol. 2003. PMID: 12706986
Similar articles
-
Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa.Semin Immunol. 2007 Apr;19(2):70-83. doi: 10.1016/j.smim.2007.04.002. Epub 2007 May 7. Semin Immunol. 2007. PMID: 17485224 Review.
-
Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice.J Biol Chem. 1997 Sep 19;272(38):23729-40. doi: 10.1074/jbc.272.38.23729. J Biol Chem. 1997. PMID: 9295317
-
The enteric microbiota regulates jejunal Paneth cell number and function without impacting intestinal stem cells.Gut Microbes. 2019;10(1):45-58. doi: 10.1080/19490976.2018.1474321. Epub 2018 Jul 11. Gut Microbes. 2019. PMID: 29883265 Free PMC article.
-
Activation of Paneth cell alpha-defensins in mouse small intestine.J Biol Chem. 2002 Feb 15;277(7):5219-28. doi: 10.1074/jbc.M109410200. Epub 2001 Dec 3. J Biol Chem. 2002. PMID: 11733520
-
Paneth cell alpha-defensins: peptide mediators of innate immunity in the small intestine.Springer Semin Immunopathol. 2005 Sep;27(2):133-46. doi: 10.1007/s00281-005-0202-x. Epub 2005 Jun 2. Springer Semin Immunopathol. 2005. PMID: 15931529 Review.
Cited by
-
Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine.Nutrients. 2014 Dec 24;7(1):45-73. doi: 10.3390/nu7010045. Nutrients. 2014. PMID: 25545102 Free PMC article. Review.
-
Circadian Host-Microbiome Interactions in Immunity.Front Immunol. 2020 Aug 14;11:1783. doi: 10.3389/fimmu.2020.01783. eCollection 2020. Front Immunol. 2020. PMID: 32922391 Free PMC article. Review.
-
TLR9 is important for protection against intestinal damage and for intestinal repair.Sci Rep. 2012;2:574. doi: 10.1038/srep00574. Epub 2012 Aug 14. Sci Rep. 2012. PMID: 22893852 Free PMC article.
-
Exploring host-microbiota interactions in animal models and humans.Genes Dev. 2013 Apr 1;27(7):701-18. doi: 10.1101/gad.212522.112. Genes Dev. 2013. PMID: 23592793 Free PMC article. Review.
-
Metaproteomics reveals functional shifts in microbial and human proteins during a preterm infant gut colonization case.Proteomics. 2015 Oct;15(20):3463-73. doi: 10.1002/pmic.201400563. Epub 2015 Jul 21. Proteomics. 2015. PMID: 26077811 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

