Modulation of fibroblast morphology and adhesion during collagen matrix remodeling
- PMID: 12429835
- PMCID: PMC133603
- DOI: 10.1091/mbc.e02-05-0291
Modulation of fibroblast morphology and adhesion during collagen matrix remodeling
Abstract
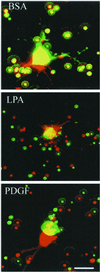
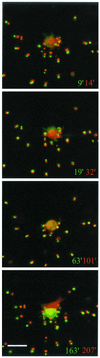
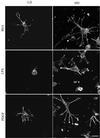
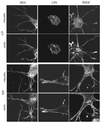
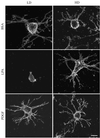
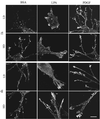
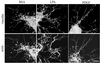
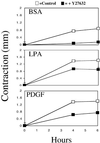
When fibroblasts are placed within a three-dimensional collagen matrix, cell locomotion results in translocation of the flexible collagen fibrils of the matrix, a remodeling process that has been implicated in matrix morphogenesis during development and wound repair. In the current experiments, we studied formation and maturation of cell-matrix interactions under conditions in which we could distinguish local from global matrix remodeling. Local remodeling was measured by the movement of collagen-embedded beads towards the cells. Global remodeling was measured by matrix contraction. Our observations show that no direct relationship occurs between protrusion and retraction of cell extensions and collagen matrix remodeling. As fibroblasts globally remodel the collagen matrix, however, their overall morphology changes from dendritic to stellate/bipolar, and cell-matrix interactions mature from punctate to focal adhesion organization. The less well organized sites of cell-matrix interaction are sufficient for translocating collagen fibrils, and focal adhesions only form after a high degree of global remodeling occurs in the presence of growth factors. Rho kinase activity is required for maturation of fibroblast morphology and formation of focal adhesions but not for translocation of collagen fibrils.
Figures




Similar articles
-
Transforming growth factor-beta1 stimulates collagen matrix remodeling through increased adhesive and contractive potential by human renal fibroblasts.Biochim Biophys Acta. 2004 Aug 23;1693(2):91-100. doi: 10.1016/j.bbamcr.2004.05.005. Biochim Biophys Acta. 2004. PMID: 15313011
-
Dendritic fibroblasts in three-dimensional collagen matrices.Mol Biol Cell. 2003 Feb;14(2):384-95. doi: 10.1091/mbc.e02-08-0493. Mol Biol Cell. 2003. PMID: 12589041 Free PMC article.
-
Direct correlation of collagen matrix deformation with focal adhesion dynamics in living corneal fibroblasts.J Cell Sci. 2003 Apr 15;116(Pt 8):1481-91. doi: 10.1242/jcs.00357. J Cell Sci. 2003. PMID: 12640033
-
Fibroblasts in three dimensional matrices: cell migration and matrix remodeling.Exp Mol Med. 2009 Dec 31;41(12):858-65. doi: 10.3858/emm.2009.41.12.096. Exp Mol Med. 2009. PMID: 19745603 Free PMC article. Review.
-
Fibroblast biology in three-dimensional collagen matrices.Trends Cell Biol. 2003 May;13(5):264-9. doi: 10.1016/s0962-8924(03)00057-6. Trends Cell Biol. 2003. PMID: 12742170 Review.
Cited by
-
Electrical stimulation of biofidelic engineered muscle enhances myotube size, force, fatigue resistance, and induces a fast-to-slow-phenotype shift.Physiol Rep. 2024 Oct;12(19):e70051. doi: 10.14814/phy2.70051. Physiol Rep. 2024. PMID: 39384537 Free PMC article.
-
Exogenous extracellular matrix proteins decrease cardiac fibroblast activation in stiffening microenvironment through CAPG.J Mol Cell Cardiol. 2021 Oct;159:105-119. doi: 10.1016/j.yjmcc.2021.06.001. Epub 2021 Jun 10. J Mol Cell Cardiol. 2021. PMID: 34118218 Free PMC article.
-
Assessment of cell viability in a three-dimensional enzymatically cross-linked collagen scaffold.J Mater Sci Mater Med. 2007 Oct;18(10):1991-2001. doi: 10.1007/s10856-007-3091-9. Epub 2007 Jun 7. J Mater Sci Mater Med. 2007. PMID: 17554605
-
Boundary stiffness regulates fibroblast behavior in collagen gels.Ann Biomed Eng. 2010 Mar;38(3):658-73. doi: 10.1007/s10439-009-9856-1. Epub 2009 Dec 10. Ann Biomed Eng. 2010. PMID: 20012205 Free PMC article.
-
Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns.Biophys J. 2005 Jul;89(1):635-50. doi: 10.1529/biophysj.105.061994. Epub 2005 Apr 22. Biophys J. 2005. PMID: 15849239 Free PMC article.
References
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol. 1998;175:323–332. - PubMed
-
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

