Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection
- PMID: 12417634
- PMCID: PMC2194099
- DOI: 10.1084/jem.20021183
Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection
Abstract
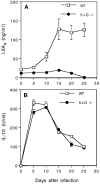
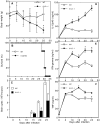
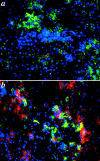
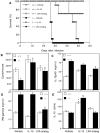
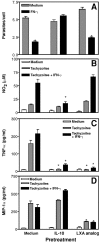
The production of interleukin (IL)-12 is critical for the development of interferon (IFN)-gamma-dependent resistance to Toxoplasma gondii. Nevertheless, when this response is dysregulated, such as occurs in the absence of IL-10, the uncontrolled inflammation that results can have lethal consequences for the host. Recently, we demonstrated that lipoxin (LX)A(4), an eicosanoid mediator that depends on 5-lipoxygenase (LO) for its biosynthesis, exerts a regulatory role on dendritic cell IL-12 production triggered artificially by a T. gondii extract. We now formally establish the physiological relevance of this pathway in the systemic control of IL-12 production induced by live T. gondii infection and demonstrate its function to be distinct from that of IL-10. Thus, T. gondii-exposed wild-type, but not 5-LO-deficient animals, produced high levels of serum LXA(4) beginning at the onset of chronic infection. Moreover, 5-LO(-/-), in contrast to wild-type mice, succumbed during the same period displaying a marked encephalitis. The increased mortality of the 5-LO(-/-) animals was also associated with significant elevations of IL-12 and IFN-gamma and was completely prevented by the administration of a stable LXA(4) analogue. Together, these findings demonstrate a new pathway involving the induction of host LXs for the in vivo regulation of proinflammatory responses during microbial infection.
Figures


Similar articles
-
Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity.Nat Immunol. 2002 Jan;3(1):76-82. doi: 10.1038/ni745. Epub 2001 Dec 17. Nat Immunol. 2002. PMID: 11743584
-
[Rudolf-Virchow Prize 1998. Award lecture. Toxoplasmosis: a model infection for studying systemic and intracerebral immune reactions].Verh Dtsch Ges Pathol. 1998;82:9-22. Verh Dtsch Ges Pathol. 1998. PMID: 10095413 German.
-
IL-10 is not required to prevent immune hyperactivity during memory responses to Toxoplasma gondii.Parasite Immunol. 2004 May;26(5):229-36. doi: 10.1111/j.0141-9838.2004.00704.x. Parasite Immunol. 2004. PMID: 15491472
-
From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii.FEMS Immunol Med Microbiol. 2003 Dec 5;39(3):193-203. doi: 10.1016/S0928-8244(03)00279-7. FEMS Immunol Med Microbiol. 2003. PMID: 14642303 Review.
-
Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function.Immunobiology. 1999 Dec;201(2):240-7. doi: 10.1016/S0171-2985(99)80064-3. Immunobiology. 1999. PMID: 10631573 Review.
Cited by
-
Narrative Review of n-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation.Nutrients. 2021 Feb 18;13(2):662. doi: 10.3390/nu13020662. Nutrients. 2021. PMID: 33670710 Free PMC article. Review.
-
Specialized Pro-Resolving Lipid Mediators: Endogenous Roles and Pharmacological Activities in Infections.Molecules. 2023 Jun 27;28(13):5032. doi: 10.3390/molecules28135032. Molecules. 2023. PMID: 37446699 Free PMC article. Review.
-
Crotoxin Modulates Macrophage Phenotypic Reprogramming.Toxins (Basel). 2023 Oct 17;15(10):616. doi: 10.3390/toxins15100616. Toxins (Basel). 2023. PMID: 37888647 Free PMC article.
-
The role of pro-resolution lipid mediators in infectious disease.Immunology. 2014 Feb;141(2):166-73. doi: 10.1111/imm.12206. Immunology. 2014. PMID: 24400794 Free PMC article. Review.
-
Use and abuse of dendritic cells by Toxoplasma gondii.Virulence. 2012 Nov 15;3(7):678-89. doi: 10.4161/viru.22833. Epub 2012 Nov 15. Virulence. 2012. PMID: 23221473 Free PMC article. Review.
References
-
- Shevach, E.M., J.T. Chang, and B.M. Segal. 1999. The critical role of IL-12 and the IL-12R beta 2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer Semin. Immunopathol. 21:249–262. - PubMed
-
- Yap, G.S., and A. Sher. 1999. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 201:240–247. - PubMed
-
- Gazzinelli, R.T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157:798–805. - PubMed
-
- Suzuki, Y., A. Sher, G. Yap, D. Park, L.E. Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375–5382. - PubMed
-
- Hoffmann, K.F., A.W. Cheever, and T.A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406–6416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

