The membrane-proximal domain of vesicular stomatitis virus G protein functions as a membrane fusion potentiator and can induce hemifusion
- PMID: 12414970
- PMCID: PMC136858
- DOI: 10.1128/jvi.76.23.12300-12311.2002
The membrane-proximal domain of vesicular stomatitis virus G protein functions as a membrane fusion potentiator and can induce hemifusion
Abstract
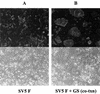
Recently we showed that the membrane-proximal stem region of the vesicular stomatitis virus (VSV) G protein ectodomain (G stem [GS]), together with the transmembrane and cytoplasmic domains, was sufficient to mediate efficient VSV budding (C. S. Robison and M. A. Whitt, J. Virol. 74:2239-2246, 2000). Here, we show that GS can also potentiate the membrane fusion activity of heterologous viral fusion proteins when GS is coexpressed with those proteins. For some fusion proteins, there was as much as a 40-fold increase in syncytium formation when GS was coexpressed compared to that seen when the fusion protein was expressed alone. Fusion potentiation by GS was not protein specific, since it occurred with both pH-dependent as well as pH-independent fusion proteins. Using a recombinant vesicular stomatitis virus encoding GS that contained an N-terminal hemagglutinin (HA) tag (GS(HA) virus), we found that the GS(HA) virus bound to cells as well as the wild-type virus did at pH 7.0; however, the GS(HA) virus was noninfectious. Analysis of cells expressing GS(HA) in a three-color membrane fusion assay revealed that GS(HA) could induce lipid mixing but not cytoplasmic mixing, indicating that GS can induce hemifusion. Treatment of GS(HA) virus-bound cells with the membrane-destabilizing drug chlorpromazine rescued the hemifusion block and allowed entry and subsequent replication of GS(HA) virus, demonstrating that GS-mediated hemifusion was a functional intermediate in the membrane fusion pathway. Using a series of truncation mutants, we also determined that only 14 residues of GS, together with the VSV G transmembrane and cytoplasmic tail, were sufficient for fusion potentiation. To our knowledge, this is the first report which shows that a small domain of one viral glycoprotein can promote the fusion activity of other, unrelated viral glycoproteins.
Figures






Similar articles
-
The membrane-proximal region of vesicular stomatitis virus glycoprotein G ectodomain is critical for fusion and virus infectivity.J Virol. 2003 Dec;77(23):12807-18. doi: 10.1128/jvi.77.23.12807-12818.2003. J Virol. 2003. PMID: 14610202 Free PMC article.
-
A recombinant vesicular stomatitis virus bearing a lethal mutation in the glycoprotein gene uncovers a second site suppressor that restores fusion.J Virol. 2011 Aug;85(16):8105-15. doi: 10.1128/JVI.00735-11. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680501 Free PMC article.
-
Important role for the transmembrane domain of severe acute respiratory syndrome coronavirus spike protein during entry.J Virol. 2006 Feb;80(3):1302-10. doi: 10.1128/JVI.80.3.1302-1310.2006. J Virol. 2006. PMID: 16415007 Free PMC article.
-
Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited.Cell Mol Life Sci. 2008 Jun;65(11):1716-28. doi: 10.1007/s00018-008-7534-3. Cell Mol Life Sci. 2008. PMID: 18345480 Free PMC article. Review.
-
Viruses as model systems in cell biology.Methods Cell Biol. 1994;43 Pt A:3-42. doi: 10.1016/s0091-679x(08)60596-8. Methods Cell Biol. 1994. PMID: 7823868 Free PMC article. Review.
Cited by
-
A spatio-temporal analysis of matrix protein and nucleocapsid trafficking during vesicular stomatitis virus uncoating.PLoS Pathog. 2010 Jul 15;6(7):e1000994. doi: 10.1371/journal.ppat.1000994. PLoS Pathog. 2010. PMID: 20657818 Free PMC article.
-
The pre-transmembrane domain of the Autographa californica multicapsid nucleopolyhedrovirus GP64 protein is critical for membrane fusion and virus infectivity.J Virol. 2009 Nov;83(21):10993-1004. doi: 10.1128/JVI.01085-09. Epub 2009 Aug 19. J Virol. 2009. PMID: 19692475 Free PMC article.
-
Characterization of EBV gB indicates properties of both class I and class II viral fusion proteins.Virology. 2007 Nov 10;368(1):102-13. doi: 10.1016/j.virol.2007.06.031. Epub 2007 Jul 25. Virology. 2007. PMID: 17655906 Free PMC article.
-
Specific targeting of human interleukin (IL)-13 receptor α2-positive cells with lentiviral vectors displaying IL-13.Hum Gene Ther Methods. 2012 Apr;23(2):137-47. doi: 10.1089/hgtb.2012.054. Epub 2012 May 21. Hum Gene Ther Methods. 2012. PMID: 22612657 Free PMC article.
-
Hydrophobic residues that form putative fusion loops of Epstein-Barr virus glycoprotein B are critical for fusion activity.J Virol. 2007 Sep;81(17):9596-600. doi: 10.1128/JVI.00758-07. Epub 2007 Jun 6. J Virol. 2007. PMID: 17553877 Free PMC article.
References
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Blumenthal, R., A. Bali-Puri, A. Walter, D. Covell, and O. Eidelman. 1987. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J. Biol. Chem. 262:13614-13619. - PubMed
-
- Bricker, B. J., R. M. Snyder, J. W. Fox, W. A. Volk, and R. R. Wagner. 1987. Monoclonal antibodies to the glycoprotein of vesicular stomatitis virus (New Jersey serotype): a method for preliminary mapping of epitopes. Virology 161:533-540. - PubMed
-
- Broder, C. C., D. S. Dimitrov, R. Blumenthal, and E. A. Berger. 1993. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s). Virology 193:483-491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

