Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1
- PMID: 12391160
- PMCID: PMC134721
- DOI: 10.1128/MCB.22.22.7929-7941.2002
Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1
Abstract
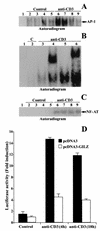
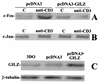
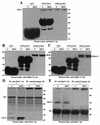
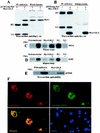
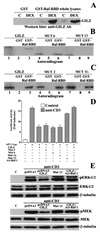
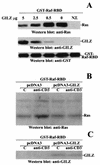
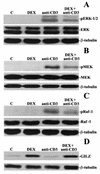
Glucocorticoid-induced leucine zipper (GILZ) is a leucine zipper protein, whose expression is augmented by dexamethasone (DEX) treatment and downregulated by T-cell receptor (TCR) triggering. Stable expression of GILZ in T cells mimics some of the effects of glucocorticoid hormones (GCH) in GCH-mediated immunosuppressive and anti-inflammatory activity. In fact, GILZ overexpression inhibits TCR-activated NF-kappaB nuclear translocation, interleukin-2 production, FasL upregulation, and the consequent activation-induced apoptosis. We have investigated the molecular mechanism underlying GILZ-mediated regulation of T-cell activation by analyzing the effects of GILZ on the activity of mitogen-activated protein kinase (MAPK) family members, including Raf, MAPK/extracellular signal-regulated kinase (ERK) 1/2 (MEK-1/2), ERK-1/2, and c-Jun NH(2)-terminal protein kinase (JNK). Our results indicate that GILZ inhibited Raf-1 phosphorylation, which resulted in the suppression of both MEK/ERK-1/2 phosphorylation and AP-1-dependent transcription. We demonstrate that GILZ interacts in vitro and in vivo with endogenous Raf-1 and that Raf-1 coimmunoprecipitated with GILZ in murine thymocytes treated with DEX. Mapping of the binding domains and experiments with GILZ mutants showed that GILZ binds the region of Raf interacting with Ras through the NH(2)-terminal region. These data suggest that GILZ contributes, through protein-to-protein interaction with Raf-1 and the consequent inhibition of Raf-MEK-ERK activation, to regulating the MAPK pathway and to providing a further mechanism underlying GCH immunosuppression.
Figures


Similar articles
-
Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ.J Biol Chem. 2001 Aug 3;276(31):29603-10. doi: 10.1074/jbc.M101522200. Epub 2001 Jun 7. J Biol Chem. 2001. PMID: 11397794
-
Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells.J Clin Invest. 2004 Feb;113(4):619-27. doi: 10.1172/JCI18975. J Clin Invest. 2004. PMID: 14966571 Free PMC article.
-
Vitamin A maintains the airway epithelium in a murine model of asthma by suppressing glucocorticoid-induced leucine zipper.Clin Exp Allergy. 2016 Jun;46(6):848-60. doi: 10.1111/cea.12646. Clin Exp Allergy. 2016. PMID: 26399569
-
Implicating the Role of GILZ in Glucocorticoid Modulation of T-Cell Activation.Front Immunol. 2019 Aug 7;10:1823. doi: 10.3389/fimmu.2019.01823. eCollection 2019. Front Immunol. 2019. PMID: 31440237 Free PMC article. Review.
-
Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action.FASEB J. 2009 Nov;23(11):3649-58. doi: 10.1096/fj.09-134684. Epub 2009 Jun 30. FASEB J. 2009. PMID: 19567371 Review.
Cited by
-
Induction of Glucocorticoid-induced Leucine Zipper (GILZ) Contributes to Anti-inflammatory Effects of the Natural Product Curcumin in Macrophages.J Biol Chem. 2016 Oct 28;291(44):22949-22960. doi: 10.1074/jbc.M116.733253. Epub 2016 Sep 14. J Biol Chem. 2016. PMID: 27629417 Free PMC article.
-
Glucocorticoid-induced leucine zipper regulates liver fibrosis by suppressing CCL2-mediated leukocyte recruitment.Cell Death Dis. 2021 Apr 29;12(5):421. doi: 10.1038/s41419-021-03704-w. Cell Death Dis. 2021. PMID: 33927191 Free PMC article.
-
Identification of actionable targets for breast cancer intervention using a diversity outbred mouse model.iScience. 2023 Mar 2;26(4):106320. doi: 10.1016/j.isci.2023.106320. eCollection 2023 Apr 21. iScience. 2023. PMID: 36968078 Free PMC article.
-
The kidneys and aldosterone/mineralocorticoid receptor system in salt-sensitive hypertension.Curr Hypertens Rep. 2011 Apr;13(2):109-15. doi: 10.1007/s11906-010-0175-6. Curr Hypertens Rep. 2011. PMID: 21207253 Free PMC article. Review.
-
GILZ as a Regulator of Cell Fate and Inflammation.Cells. 2021 Dec 30;11(1):122. doi: 10.3390/cells11010122. Cells. 2021. PMID: 35011684 Free PMC article. Review.
References
-
- Auphan, N., J. A. DiDonato, C. Rosette, A. Helmberg, and M. Karin. 1995. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270:286-290. - PubMed
-
- Ayroldi, E., G. Migliorati, S. Bruscoli, C. Marchetti, O. Zollo, L. Cannarile, F. D'Adamio, and C. Riccardi. 2001. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor κB. Blood 98:743-753. - PubMed
-
- Ayroldi, E., O. Zollo, L. Cannarile, F. D'Adamio, U. Grohmann, D. V. Delfino, and C. Riccardi. 1998. Interleukin-6 (IL-6) prevents activation-induced cell death: IL-2-independent inhibition of Fas/fasL expression and cell death. Blood 92:4212-4219. - PubMed
-
- Barnes, P. J., and I. Adcock. 1993. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol. Sci. 14:436-441. - PubMed
-
- Beato, M. 1991. Transcriptional control by nuclear receptors. FASEB J. 5:2044-2051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
