GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus
- PMID: 12388752
- PMCID: PMC129961
- DOI: 10.1091/mbc.e02-01-0004
GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus
Abstract
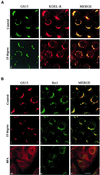
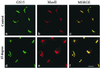
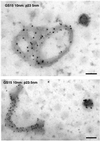
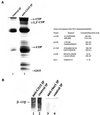
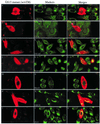
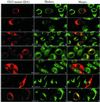
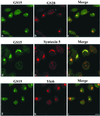
The subcellular localization, interacting partners, and function of GS15, a Golgi SNARE, remain to be established. In our present study, it is revealed that unlike proteins (Bet1 and the KDEL receptor) cycling between the Golgi and the intermediate compartment (IC, inclusive of the ER exit sites), GS15 is not redistributed into the IC upon incubation at 15 degrees C or when cells are treated with brefeldin A. Immuno-electron microscopy (immuno-EM) reveals that GS15 is mainly found in the medial-cisternae of the Golgi apparatus and adjacent tubulo-vesicular elements. Coimmunoprecipitation experiments suggest that GS15 exists in a distinct SNARE complex that contains SNAREs (syntaxin5, GS28, and Ykt6) that are implicated in both ER-to-Golgi and intra-Golgi transport but not with SNAREs involved exclusively in ER-to-Golgi traffic. Furthermore, components of COPI coat can be selectively coimmunoprecipitated with GS15 from Golgi extracts. Overexpression of mutant forms of GS15 affects the normal distribution of cis- and medial-Golgi proteins (GS28, syntaxin 5, and Golgi mannosidase II), whereas proteins of the trans-Golgi and TGN (Vti1-rp2/Vti1a and syntaxin 6) and Golgi matrix/scaffold (GM130 and p115) are less affected. When the level of GS15 is reduced by duplex 21-nt small interfering RNA (siRNA)-mediated knockdown approach, diverse markers of the Golgi apparatus are redistributed into small dotty and diffuse labeling, suggesting an essential role of GS15 in the Golgi apparatus.
Figures




Similar articles
-
Participation of the syntaxin 5/Ykt6/GS28/GS15 SNARE complex in transport from the early/recycling endosome to the trans-Golgi network.Mol Biol Cell. 2004 Sep;15(9):4011-22. doi: 10.1091/mbc.e03-12-0876. Epub 2004 Jun 23. Mol Biol Cell. 2004. PMID: 15215310 Free PMC article.
-
Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport.J Biol Chem. 2001 Jul 20;276(29):27480-7. doi: 10.1074/jbc.M102786200. Epub 2001 Apr 25. J Biol Chem. 2001. PMID: 11323436
-
Countercurrent distribution of two distinct SNARE complexes mediating transport within the Golgi stack.Mol Biol Cell. 2004 Apr;15(4):1506-18. doi: 10.1091/mbc.e03-08-0625. Epub 2004 Jan 23. Mol Biol Cell. 2004. PMID: 14742712 Free PMC article.
-
SNAREs and membrane fusion in the Golgi apparatus.Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review.
-
Diverse Role of SNARE Protein GS28 in Vesicle Trafficking and Diseases.Curr Protein Pept Sci. 2023;24(4):288-295. doi: 10.2174/1389203724666230315143542. Curr Protein Pept Sci. 2023. PMID: 36924089 Review.
Cited by
-
Cargo trafficking between endosomes and the trans-Golgi network.Histochem Cell Biol. 2013 Sep;140(3):307-15. doi: 10.1007/s00418-013-1125-6. Epub 2013 Jul 14. Histochem Cell Biol. 2013. PMID: 23851467 Review.
-
Tethering factor P115: a new model for tether-SNARE interactions.Bioarchitecture. 2012 Sep-Oct;2(5):175-80. doi: 10.4161/bioa.21702. Epub 2012 Sep 1. Bioarchitecture. 2012. PMID: 22992751 Free PMC article.
-
Golgi bypass: skirting around the heart of classical secretion.Cold Spring Harb Perspect Biol. 2011 Apr 1;3(4):a005298. doi: 10.1101/cshperspect.a005298. Cold Spring Harb Perspect Biol. 2011. PMID: 21441587 Free PMC article. Review.
-
Transcriptome analysis using patient iPSC-derived skeletal myocytes: Bet1L as a new molecule possibly linked to neuromuscular junction degeneration in ALS.Exp Neurol. 2021 Nov;345:113815. doi: 10.1016/j.expneurol.2021.113815. Epub 2021 Jul 24. Exp Neurol. 2021. PMID: 34310943 Free PMC article.
-
Comprehensive Proteomic Characterization of the Intra-Golgi Trafficking Intermediates.bioRxiv [Preprint]. 2024 Nov 12:2024.10.25.620336. doi: 10.1101/2024.10.25.620336. bioRxiv. 2024. PMID: 39484492 Free PMC article. Preprint.
References
-
- Allan BB, Balch WE. Protein sorting by directed maturation of Golgi compartments. Science. 1999;285:63–66. - PubMed
-
- Antonny B, Schekman R. ER export. public transportation by the COPII coach. Curr Opin Cell Biol. 2001;13:438–443. - PubMed
-
- Balch WE, McCaffery JM, Plunter H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;77:841–852. - PubMed
-
- Banfield DK, Lewis MJ, Pelham HR. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

