Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation
- PMID: 12388709
- PMCID: PMC136769
- DOI: 10.1128/jvi.76.22.11476-11483.2002
Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation
Abstract
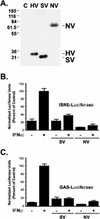
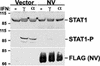
Characterization of recent outbreaks of fatal encephalitis in southeast Asia identified the causative agent to be a previously unrecognized enveloped negative-strand RNA virus of the Paramyxoviridae family, Nipah virus. One feature linking Nipah virus to this family is a conserved cysteine-rich domain that is the hallmark of paramyxovirus V proteins. The V proteins of other paramyxovirus species have been linked with evasion of host cell interferon (IFN) signal transduction and subsequent antiviral responses by inducing proteasomal degradation of the IFN-responsive transcription factors, STAT1 or STAT2. Here we demonstrate that Nipah virus V protein escapes IFN by a distinct mechanism involving direct inhibition of STAT protein function. Nipah virus V protein differs from other paramyxovirus V proteins in its subcellular distribution but not in its ability to inhibit cellular IFN responses. Nipah virus V protein does not induce STAT degradation but instead inhibits IFN responses by forming high-molecular-weight complexes with both STAT1 and STAT2. We demonstrate that Nipah virus V protein accumulates in the cytoplasm by a Crm1-dependent mechanism, alters the STAT protein subcellular distribution in the steady state, and prevents IFN-stimulated STAT redistribution. Consistent with the formation of complexes, STAT protein tyrosine phosphorylation is inhibited in cells expressing the Nipah virus V protein. As a result, Nipah virus V protein efficiently prevents STAT1 and STAT2 nuclear translocation in response to IFN, inhibiting cellular responses to both IFN-alpha and IFN-gamma.
Figures
Similar articles
-
Identification of the nuclear export signal and STAT-binding domains of the Nipah virus V protein reveals mechanisms underlying interferon evasion.J Virol. 2004 May;78(10):5358-67. doi: 10.1128/jvi.78.10.5358-5367.2004. J Virol. 2004. PMID: 15113915 Free PMC article.
-
Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation.J Virol. 2003 Nov;77(21):11842-5. doi: 10.1128/jvi.77.21.11842-11845.2003. J Virol. 2003. PMID: 14557668 Free PMC article.
-
Selective STAT protein degradation induced by paramyxoviruses requires both STAT1 and STAT2 but is independent of alpha/beta interferon signal transduction.J Virol. 2002 May;76(9):4190-8. doi: 10.1128/jvi.76.9.4190-4198.2002. J Virol. 2002. PMID: 11932384 Free PMC article.
-
Host evasion by emerging paramyxoviruses: Hendra virus and Nipah virus v proteins inhibit interferon signaling.Viral Immunol. 2004;17(2):210-9. doi: 10.1089/0882824041310568. Viral Immunol. 2004. PMID: 15279700 Review.
-
Paramyxovirus strategies for evading the interferon response.Rev Med Virol. 2002 Nov-Dec;12(6):337-57. doi: 10.1002/rmv.357. Rev Med Virol. 2002. PMID: 12410527 Review.
Cited by
-
TRIM Proteins in Host Defense and Viral Pathogenesis.Curr Clin Microbiol Rep. 2020;7(4):101-114. doi: 10.1007/s40588-020-00150-8. Epub 2020 Aug 8. Curr Clin Microbiol Rep. 2020. PMID: 32837832 Free PMC article. Review.
-
Inhibition of interferon response by cystatin B: implication in HIV replication of macrophage reservoirs.J Neurovirol. 2012 Feb;18(1):20-9. doi: 10.1007/s13365-011-0061-2. Epub 2011 Dec 7. J Neurovirol. 2012. PMID: 22147503 Free PMC article.
-
Early induction of interferon-responsive mRNAs in Creutzfeldt-Jakob disease.J Neurovirol. 2004 Feb;10(1):29-40. doi: 10.1080/13550280490261761. J Neurovirol. 2004. PMID: 14982726 Free PMC article.
-
STAT3 ubiquitylation and degradation by mumps virus suppress cytokine and oncogene signaling.J Virol. 2003 Jun;77(11):6385-93. doi: 10.1128/jvi.77.11.6385-6393.2003. J Virol. 2003. PMID: 12743296 Free PMC article.
-
Nipah virus: an emergent paramyxovirus causing severe encephalitis in humans.J Neurovirol. 2005 Oct;11(5):481-7. doi: 10.1080/13550280500187435. J Neurovirol. 2005. PMID: 16287690 Review.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. - PubMed
-
- Chua, K. B., W. J. Bellini, P. A. Rota, B. H. Harcourt, A. Tamin, S. K. Lam, T. G. Ksiazek, P. E. Rollin, S. R. Zaki, W. Shieh, C. S. Goldsmith, D. J. Gubler, J. T. Roehrig, B. Eaton, A. R. Gould, J. Olson, H. Field, P. Daniels, A. E. Ling, C. J. Peters, L. J. Anderson, and B. W. Mahy. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288:1432-1435. - PubMed
-
- Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

