Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes
- PMID: 12356885
- PMCID: PMC2290557
- DOI: 10.1113/jphysiol.2002.022574
Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes
Abstract
In the present study we have investigated the role of diacylglycerol (DAG) and protein kinase C (PKC) in mediating activation of Ca(2+)-permeable store-operated channels (SOCs) by noradrenaline in rabbit portal vein smooth muscle cells. With cell-attached recording, bath application of noradrenaline, 1-oleoyl-acetyl-sn-glycerol (OAG) and phorbol 12,13-dibutyrate (PDBu) evoked single channel currents. The biophysical properties of these channel currents were similar to those of the channel currents activated by depletion of internal Ca(2+) stores with cyclopiazonic acid (CPA). The activation of SOCs in cell-attached recording by noradrenaline, OAG, PDBu, CPA and the acetoxymethyl ester form of BAPTA (BAPTA-AM) was markedly inhibited by the PKC inhibitors chelerythrine and RO-31-8220. In isolated outside-out patches CPA did not evoke SOCs but noradrenaline stimulated SOC activity, which was reduced by about 90 % by PKC inhibitors. The addition of the serine/threonine phosphatase inhibitors calyculin A and microcystin also stimulated SOCs in isolated outside-out patches. It is concluded that in rabbit portal vein myocytes, noradrenaline activates SOCs via DAG and PKC, possibly by a store-independent mechanism. In addition in this cell type it appears that PKC and phosphorylation may play an important role in stimulating SOC activity in response to depletion of internal Ca(2+) stores by CPA and BAPTA-AM.
Figures

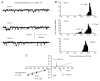
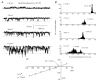




Comment in
-
SOCs - store-operated channels in vascular smooth muscle?J Physiol. 2002 Oct 1;544(Pt 1):1. doi: 10.1113/jphysiol.2002.027151. J Physiol. 2002. PMID: 12356874 Free PMC article. No abstract available.
Similar articles
-
Stimulation of beta-adrenoceptors inhibits store-operated channel currents via a cAMP-dependent protein kinase mechanism in rabbit portal vein myocytes.J Physiol. 2005 Jan 15;562(Pt 2):395-406. doi: 10.1113/jphysiol.2004.077602. Epub 2004 Nov 4. J Physiol. 2005. PMID: 15528235 Free PMC article.
-
Facilitatory effect of Ins(1,4,5)P3 on store-operated Ca2+-permeable cation channels in rabbit portal vein myocytes.J Physiol. 2005 Jul 1;566(Pt 1):161-71. doi: 10.1113/jphysiol.2005.088260. Epub 2005 Apr 28. J Physiol. 2005. PMID: 15860523 Free PMC article.
-
Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes.J Physiol. 2003 Nov 1;552(Pt 3):789-95. doi: 10.1113/jphysiol.2003.052977. Epub 2003 Sep 12. J Physiol. 2003. PMID: 12972630 Free PMC article.
-
Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells.Cell Calcium. 2003 May-Jun;33(5-6):345-56. doi: 10.1016/s0143-4160(03)00048-4. Cell Calcium. 2003. PMID: 12765681 Review.
-
Receptor-operated Ca2(+)-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective.J Cardiovasc Electrophysiol. 2002 May;13(5):493-501. doi: 10.1046/j.1540-8167.2002.00493.x. J Cardiovasc Electrophysiol. 2002. PMID: 12030534 Review.
Cited by
-
A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes.J Physiol. 2002 Feb 1;538(Pt 3):717-28. doi: 10.1113/jphysiol.2001.013101. J Physiol. 2002. PMID: 11826160 Free PMC article.
-
Diabetes-induced activation of protein kinase C inhibits store-operated Ca2+ uptake in rat retinal microvascular smooth muscle.Diabetologia. 2003 Sep;46(9):1252-9. doi: 10.1007/s00125-003-1178-5. Epub 2003 Jul 30. Diabetologia. 2003. PMID: 12898009
-
Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1079-90. doi: 10.1152/ajpcell.00548.2009. Epub 2010 Aug 25. Am J Physiol Cell Physiol. 2010. PMID: 20739625 Free PMC article.
-
On the activation mechanism of store-operated calcium channels.Pflugers Arch. 2006 Dec;453(3):303-11. doi: 10.1007/s00424-006-0089-y. Epub 2006 Jun 21. Pflugers Arch. 2006. PMID: 16944196 Review.
-
Altered protein kinase C regulation of pulmonary endothelial store- and receptor-operated Ca2+ entry after chronic hypoxia.J Pharmacol Exp Ther. 2010 Sep 1;334(3):753-60. doi: 10.1124/jpet.110.165563. Epub 2010 Jun 24. J Pharmacol Exp Ther. 2010. PMID: 20576798 Free PMC article.
References
-
- Broad LM, Braun F-J, Lieveremont J-P, Bird G, Kurosaki T, Putney JW. Role of the phospholipase-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calium entry. Journal of Biological Chemistry. 2001;276:15945–15952. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

