Promoter sequences required for reactivation of Epstein-Barr virus from latency
- PMID: 12239304
- PMCID: PMC136560
- DOI: 10.1128/jvi.76.20.10282-10289.2002
Promoter sequences required for reactivation of Epstein-Barr virus from latency
Abstract
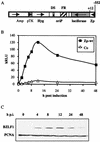
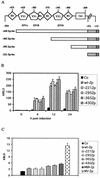
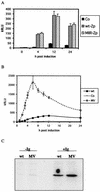
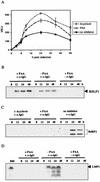
A luciferase reporter system with stably transfected oriP plasmids in Akata Burkitt's lymphoma cells provides a quantitative assay for the BZLF1 Zp promoter in response to B-cell receptor (BCR) activation by cross-linking with anti-immunoglobulin. In this system, detailed kinetic studies of promoter activity are possible. Previously reported promoter elements upstream of -221 from the transcription start and the ZIIR sequence had little effect on the Zp promoter, but the ZI and ZIIIA elements were essential for early activation. The ZIIIB element mediates autoactivation. Mutation of the ZV repressor sequence greatly increased the induction of the promoter but did not make it constitutively active. Zp transcription in response to BCR cross-linking declined after a few hours; this decline was reduced and delayed by acyclovir or phosphonoacetic acid, indicating that viral DNA replication or a late viral gene can play a role in the switch off of the Zp promoter. Late expression of the LMP1 protein may account for this.
Figures


Similar articles
-
Signal Transduction and Transcription Factor Modification during Reactivation of Epstein-Barr Virus from Latency.J Virol. 2002 Oct;76(20):10290-8. doi: 10.1128/jvi.76.20.10290-10298.2002. J Virol. 2002. PMID: 12239305 Free PMC article.
-
Lytic cycle gene regulation of Epstein-Barr virus.J Virol. 2004 Dec;78(24):13460-9. doi: 10.1128/JVI.78.24.13460-13469.2004. J Virol. 2004. PMID: 15564457 Free PMC article.
-
The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells.Mol Carcinog. 2002 Mar;33(3):137-45. doi: 10.1002/mc.10029. Mol Carcinog. 2002. PMID: 11870879
-
Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene.Trends Microbiol. 1997 Oct;5(10):399-405. doi: 10.1016/S0966-842X(97)01129-3. Trends Microbiol. 1997. PMID: 9351176 Review.
-
Regulation of Epstein-Barr virus reactivation from latency.Microbiol Immunol. 2014 Jun;58(6):307-17. doi: 10.1111/1348-0421.12155. Microbiol Immunol. 2014. PMID: 24786491 Review.
Cited by
-
The B-cell specific transcription factor, Oct-2, promotes Epstein-Barr virus latency by inhibiting the viral immediate-early protein, BZLF1.PLoS Pathog. 2012 Feb;8(2):e1002516. doi: 10.1371/journal.ppat.1002516. Epub 2012 Feb 9. PLoS Pathog. 2012. PMID: 22346751 Free PMC article.
-
Epigenetic lifestyle of Epstein-Barr virus.Semin Immunopathol. 2020 Apr;42(2):131-142. doi: 10.1007/s00281-020-00792-2. Epub 2020 Mar 30. Semin Immunopathol. 2020. PMID: 32232535 Free PMC article. Review.
-
Either ZEB1 or ZEB2/SIP1 can play a central role in regulating the Epstein-Barr virus latent-lytic switch in a cell-type-specific manner.J Virol. 2010 Jun;84(12):6139-52. doi: 10.1128/JVI.02706-09. Epub 2010 Apr 7. J Virol. 2010. PMID: 20375168 Free PMC article.
-
De novo protein synthesis is required for lytic cycle reactivation of Epstein-Barr virus, but not Kaposi's sarcoma-associated herpesvirus, in response to histone deacetylase inhibitors and protein kinase C agonists.J Virol. 2007 Sep;81(17):9279-91. doi: 10.1128/JVI.00982-07. Epub 2007 Jun 27. J Virol. 2007. PMID: 17596302 Free PMC article.
-
Signal Transduction and Transcription Factor Modification during Reactivation of Epstein-Barr Virus from Latency.J Virol. 2002 Oct;76(20):10290-8. doi: 10.1128/jvi.76.20.10290-10298.2002. J Virol. 2002. PMID: 12239305 Free PMC article.
References
-
- Bauer, G., P. Hofler, and M. Simon. 1982. Epstein-Barr virus induction by a serum factor. Characterization of the purified factor and the mechanism of its activation. J. Biol. Chem. 257:11411-11415. - PubMed
-
- Corcoran, E. E., and A. R. Means. 2001. Defining Ca2+/calmodulin-dependent protein kinase cascades in transcriptional regulation. J. Biol. Chem. 276:2975-2978. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

