Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery
- PMID: 12186903
- PMCID: PMC136464
- DOI: 10.1128/jvi.76.18.9194-9206.2002
Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery
Abstract
During the early phase of infection, the E1B-55K protein of adenovirus type 5 (Ad5) counters the E1A-induced stabilization of p53, whereas in the late phase, E1B-55K modulates the preferential nucleocytoplasmic transport and translation of the late viral mRNAs. The mechanism(s) by which E1B-55K performs these functions has not yet been clearly elucidated. In this study, we have taken a proteomics-based approach to identify and characterize novel E1B-55K-associated proteins. A multiprotein E1B-55K-containing complex was immunopurified from Ad5-infected HeLa cells and found to contain E4-orf6, as well as several cellular factors previously implicated in the ubiquitin-proteasome-mediated destruction of proteins, including Cullin-5, Rbx1/ROC1/Hrt1, and Elongins B and C. We further demonstrate that a complex containing these as well as other proteins is capable of directing the polyubiquitination of p53 in vitro. These ubiquitin ligase components were found in a high-molecular-mass complex of 800 to 900 kDa. We propose that these newly identified binding partners (Cullin-5, Elongins B and C, and Rbx1) complex with E1B-55K and E4-orf6 during Ad infection to form part of an E3 ubiquitin ligase that targets specific protein substrates for degradation. We further suggest that E1B-55K functions as the principal substrate recognition component of this SCF-type ubiquitin ligase, whereas E4-orf6 may serve to nucleate the assembly of the complex. Lastly, we describe the identification and characterization of two novel E1B-55K interacting factors, importin-alpha 1 and pp32, that may also participate in the functions previously ascribed to E1B-55K and E4-orf6.
Figures

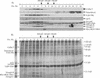
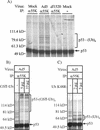

Similar articles
-
Adenovirus E4-ORF3 Targets PIAS3 and Together with E1B-55K Remodels SUMO Interactions in the Nucleus and at Virus Genome Replication Domains.J Virol. 2015 Oct;89(20):10260-72. doi: 10.1128/JVI.01091-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223632 Free PMC article.
-
Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export.J Virol. 2007 Jan;81(2):575-87. doi: 10.1128/JVI.01725-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079297 Free PMC article.
-
Adenovirus E4 34k and E1b 55k oncoproteins target host DNA ligase IV for proteasomal degradation.J Virol. 2007 Jul;81(13):7034-40. doi: 10.1128/JVI.00029-07. Epub 2007 Apr 25. J Virol. 2007. PMID: 17459921 Free PMC article.
-
Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus.Oncogene. 2005 Nov 21;24(52):7673-85. doi: 10.1038/sj.onc.1209040. Oncogene. 2005. PMID: 16299528 Review.
-
Regulation of mRNA production by the adenoviral E1B 55-kDa and E4 Orf6 proteins.Curr Top Microbiol Immunol. 2003;272:287-330. doi: 10.1007/978-3-662-05597-7_10. Curr Top Microbiol Immunol. 2003. PMID: 12747554 Review.
Cited by
-
Adenovirus E4-ORF3 Targets PIAS3 and Together with E1B-55K Remodels SUMO Interactions in the Nucleus and at Virus Genome Replication Domains.J Virol. 2015 Oct;89(20):10260-72. doi: 10.1128/JVI.01091-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223632 Free PMC article.
-
Adenovirus-mediated ubiquitination alters protein-RNA binding and aids viral RNA processing.Nat Microbiol. 2020 Oct;5(10):1217-1231. doi: 10.1038/s41564-020-0750-9. Epub 2020 Jul 13. Nat Microbiol. 2020. PMID: 32661314 Free PMC article.
-
The human adenovirus type 5 E1B 55 kDa protein obstructs inhibition of viral replication by type I interferon in normal human cells.PLoS Pathog. 2012;8(8):e1002853. doi: 10.1371/journal.ppat.1002853. Epub 2012 Aug 9. PLoS Pathog. 2012. PMID: 22912576 Free PMC article.
-
Adenovirus E1B 55-kilodalton protein is a p53-SUMO1 E3 ligase that represses p53 and stimulates its nuclear export through interactions with promyelocytic leukemia nuclear bodies.J Virol. 2010 Dec;84(23):12210-25. doi: 10.1128/JVI.01442-10. Epub 2010 Sep 22. J Virol. 2010. PMID: 20861261 Free PMC article.
-
Sequestration of p53 in the cytoplasm by adenovirus type 12 E1B 55-kilodalton oncoprotein is required for inhibition of p53-mediated apoptosis.J Virol. 2003 Dec;77(24):13171-81. doi: 10.1128/jvi.77.24.13171-13181.2003. J Virol. 2003. PMID: 14645574 Free PMC article.
References
-
- Bai, J., J. R. Brody, S. S. Kadkol, and G. R. Pasternack. 2001. Tumor suppression and potentiation by manipulation of pp32 expression. Oncogene 20:2153-2160. - PubMed
-
- Barker, D. D., and A. J. Berk. 1987. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology 156:107-121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

