MHC heterozygosity confers a selective advantage against multiple-strain infections
- PMID: 12177415
- PMCID: PMC123244
- DOI: 10.1073/pnas.162006499
MHC heterozygosity confers a selective advantage against multiple-strain infections
Abstract
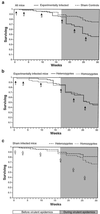
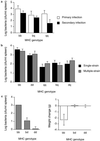
Genetic heterozygosity is thought to enhance resistance of hosts to infectious diseases, but few tests of this idea exist. In particular, heterozygosity at the MHC, the highly polymorphic loci that control immunological recognition of pathogens, is suspected to confer a selective advantage by enhancing resistance to infectious diseases (the "heterozygote advantage" hypothesis). To test this hypothesis, we released mice into large population enclosures and challenged them with multiple strains of Salmonella and one of Listeria. We found that during Salmonella infections with three avirulent strains, MHC heterozygotes had greater survival and weight than homozygotes (unlike sham controls), and they were more likely to clear chronic Salmonella infection than homozygotes. In laboratory experiments, we found that MHC heterozygosity enhanced the clearance of multiple-strain Salmonella infections. Yet, contrary to what is widely assumed, the benefits of heterozygosity were due to resistance being dominant rather than overdominant, i.e., heterozygotes were more resistant than the average of parental homozygotes, but they were not more resistant than both. The fact that MHC heterozygotes were more resistant to infection and had higher fitness than homozygotes provides a functional explanation for MHC-disassortative mating preferences.
Figures

Similar articles
-
Major histocompatibility complex heterozygosity reduces fitness in experimentally infected mice.Genetics. 2007 Aug;176(4):2501-8. doi: 10.1534/genetics.107.074815. Epub 2007 Jul 1. Genetics. 2007. PMID: 17603099 Free PMC article.
-
Major histocompatibility complex heterozygote superiority during coinfection.Infect Immun. 2003 Apr;71(4):2079-86. doi: 10.1128/IAI.71.4.2079-2086.2003. Infect Immun. 2003. PMID: 12654829 Free PMC article.
-
Infection-dependent phenotypes in MHC-congenic mice are not due to MHC: can we trust congenic animals?BMC Immunol. 2004 Jul 9;5:14. doi: 10.1186/1471-2172-5-14. BMC Immunol. 2004. PMID: 15245582 Free PMC article.
-
Parasites and individual major histocompatibility complex diversity--an optimal choice?Microbes Infect. 2004 Oct;6(12):1110-6. doi: 10.1016/j.micinf.2004.05.025. Microbes Infect. 2004. PMID: 15380781 Review.
-
The nature of selection on the major histocompatibility complex.Crit Rev Immunol. 1997;17(2):179-224. doi: 10.1615/critrevimmunol.v17.i2.40. Crit Rev Immunol. 1997. PMID: 9094452 Review.
Cited by
-
A sequence-based approach demonstrates that balancing selection in classical human leukocyte antigen (HLA) loci is asymmetric.Hum Mol Genet. 2013 Jan 15;22(2):252-61. doi: 10.1093/hmg/dds424. Epub 2012 Oct 12. Hum Mol Genet. 2013. PMID: 23065702 Free PMC article.
-
Meta-analysis of major histocompatibility complex (MHC) class IIA reveals polymorphism and positive selection in many vertebrate species.Mol Ecol. 2022 Dec;31(24):6390-6406. doi: 10.1111/mec.16726. Epub 2022 Oct 19. Mol Ecol. 2022. PMID: 36208104 Free PMC article. Review.
-
Evolution of MHC class I genes in the European badger (Meles meles).Ecol Evol. 2012 Jul;2(7):1644-62. doi: 10.1002/ece3.285. Ecol Evol. 2012. PMID: 22957169 Free PMC article.
-
Molecular Characterization of MHC Class I Genes in Four Species of the Turdidae Family to Assess Genetic Diversity and Selection.Biomed Res Int. 2021 Apr 10;2021:5585687. doi: 10.1155/2021/5585687. eCollection 2021. Biomed Res Int. 2021. PMID: 33937397 Free PMC article.
-
A single genomic region involving a putative chromosome rearrangement in flat oyster (Ostrea edulis) is associated with differential host resilience to the parasite Bonamia ostreae.Evol Appl. 2022 Jul 21;15(9):1408-1422. doi: 10.1111/eva.13446. eCollection 2022 Sep. Evol Appl. 2022. PMID: 36187184 Free PMC article.
References
-
- Falk K., Rotzschke, O., Stevanovic, S., Jung, G. & Rammensee, H. G. (1991) Nature (London) 351, 290-296. - PubMed
-
- Doherty P. C. & Zinkernagel, R. M. (1975) Nature (London) 256, 50-52. - PubMed
-
- Dyall R., Messaoudi, I., Janetzki, S., Nikolic, Z. & Ugic, J. (2000) J. Immunol. 164, 1695-1698. - PubMed
-
- Apanius V., Penn, D., Slev, P., Ruff, L. R. & Potts, W. K. (1997) Crit. Rev. Immunol. 17, 179-224. - PubMed
-
- Penn D. J. (2002) Ethology 108, 1-21.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

