Hepatoprotective role of Ganoderma lucidum polysaccharide against BCG-induced immune liver injury in mice
- PMID: 12174387
- PMCID: PMC4656329
- DOI: 10.3748/wjg.v8.i4.728
Hepatoprotective role of Ganoderma lucidum polysaccharide against BCG-induced immune liver injury in mice
Abstract
Aim: To examine the effect of ganoderma lucidum polysaccharide (GLP) on the immune liver injury induced by BCG infection, and investigate the relationship between degrees of hepatic damage and NO production in mice.
Methods: Immune hepatic injury was markedly induced by BCG-pretreatment (125 mg.kg(-1), 2-week, iv) or by BCG-pretreatment plus lipopolysaccharide (LPS, 125 microg.kg(-1), 12-hour,iv) in mice in vivo. Hepatocellular damage induced by BCG-pretreated plus inflammatory cytokines mixture (CM), which was included TNF-alpha, IL-1beta, IFN-gamma and LPS in culture medium in vitro. Administration of GLP was performed by oral or incubating with culture medium at immune stimuli simultaneity. Liver damage was determined by activity of alanine aminotransferase (ALT) in serum and in hepatocytes cultured supernatant, by liver weight changes and histopathological examination. NO production in the cultured supernatant was determined by the Griess reaction. Moreover, inducible nitric oxide synthase (iNOS) protein expression was also examinated by immunohistochemical method.
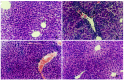
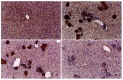
Results: Immune hepatic injury was markedly induced by BCG or BCG plus inflammatory cytokines in BALB/c mice in vivo and in vitro. Under BCG-stimulated condition, augment of the liver weight and increase of the serum/supernatant ALT level were observed, as well as granuloma forming and inflammatory cells soakage were observed by microscopic analysis within liver tissues. Moreover, NO production was also increased by BCG or/and CM stimuli in the culture supernatant, and a lot of iNOS positive staining was observed in BCG-prestimulated hepatic sections. Application of GLP significantly mitigated hepatic tumefaction, decreased ALT enzyme release and NO production in serum/supernatant, improved the pathological changes of chronic and acute inflammation induced by BCG-stimuli in mice. Moreover, the immunohistochemical result showed that GLP inhibited iNOS protein expression in BCG-immune hepatic damage model.
Conclusion: The present study indicates that NO participates in immune liver injury induced by Mycobacterium bovis BCG infection. The mechanisms of protective roles by GLP for BCG-induced immune liver injury may be due to influence NO production in mice.
Figures
Similar articles
-
Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide.World J Gastroenterol. 2004 Sep 15;10(18):2690-6. doi: 10.3748/wjg.v10.i18.2690. World J Gastroenterol. 2004. PMID: 15309720 Free PMC article.
-
Inhibition of inducible nitric oxide synthase protects against liver injury induced by mycobacterial infection and endotoxins.J Hepatol. 2004 Nov;41(5):773-81. doi: 10.1016/j.jhep.2004.07.031. J Hepatol. 2004. PMID: 15519650
-
[Effects of selective induceble nitric oxide synthase inhibitor on immunological hepatic injury in rat].Zhonghua Yi Xue Za Zhi. 1998 Jul;78(7):540-3. Zhonghua Yi Xue Za Zhi. 1998. PMID: 10923468 Chinese.
-
Inducible nitric oxide synthase in the liver: regulation and function.Biochemistry (Mosc). 1998 Jul;63(7):766-81. Biochemistry (Mosc). 1998. PMID: 9721329 Review.
-
Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action.Nutrients. 2023 Apr 13;15(8):1874. doi: 10.3390/nu15081874. Nutrients. 2023. PMID: 37111092 Free PMC article. Review.
Cited by
-
Rapid mitogen-activated protein kinase by basic fibroblast growth factor in rat intestine after ischemia/reperfusion injury.World J Gastroenterol. 2003 Jun;9(6):1312-7. doi: 10.3748/wjg.v9.i6.1312. World J Gastroenterol. 2003. PMID: 12800247 Free PMC article.
-
Ganoderma lucidum polysaccharides counteract inhibition on CD71 and FasL expression by culture supernatant of B16F10 cells upon lymphocyte activation.Exp Ther Med. 2013 Apr;5(4):1117-1122. doi: 10.3892/etm.2013.931. Epub 2013 Jan 29. Exp Ther Med. 2013. PMID: 23596479 Free PMC article.
-
Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China.J Cell Mol Med. 2018 Jul;22(7):3278-3297. doi: 10.1111/jcmm.13613. Epub 2018 Apr 24. J Cell Mol Med. 2018. PMID: 29691994 Free PMC article. Review.
-
Significance of Medicinal Mushrooms in Integrative Oncology: A Narrative Review.Front Pharmacol. 2020 Nov 11;11:580656. doi: 10.3389/fphar.2020.580656. eCollection 2020. Front Pharmacol. 2020. PMID: 33424591 Free PMC article. Review.
-
Antioxidant and preventive effects of extract from nymphaea Candida flower on in vitro immunological liver injury of rat primary hepatocyte cultures.Evid Based Complement Alternat Med. 2011;2011:497673. doi: 10.1093/ecam/nep003. Epub 2011 Jun 23. Evid Based Complement Alternat Med. 2011. PMID: 19196740 Free PMC article.
References
-
- Bao X, Liu C, Fang J, Li X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr Res. 2001;332:67–74. - PubMed
-
- Cheung WM, Hui WS, Chu PW, Chiu SW, Ip NY. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000;486:291–296. - PubMed
-
- Ma L, Lin ZB. Effects of Ganoderma polysaccharides on IL-2 production by mouse splenocytes in vitro. J Beij Med Univ. 1991;23:412–417.
-
- Lei LS, Lin ZB. Effect of Ganoderma polysaccharides on T cell subpopulations and production of interleukin 2 in mixed lymphocyte response. Yaoxue Xuebao. 1992;27:331–335. - PubMed
-
- Zhang QH, Lin ZB. The antitumor activity of Ganoderma lucidum (Curt.: Fr.) P. Karst. (Ling Zhi) (Aphyllophoromy cetideae) polysaccharides is related to tumor nerosis factor-a and interferon-γ. Inter J Med Mushrooms. 1999;1:207–215.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

