Heat-evoked activation of the ion channel, TRPV4
- PMID: 12151520
- PMCID: PMC6758176
- DOI: 10.1523/JNEUROSCI.22-15-06408.2002
Heat-evoked activation of the ion channel, TRPV4
Abstract
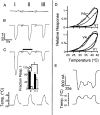
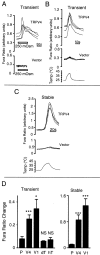
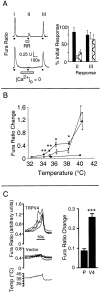
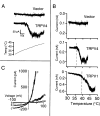
The mammalian nervous system constantly evaluates internal and environmental temperatures to maintain homeostasis and to avoid thermal extremes. Several members of the transient receptor potential (TRP) family of ion channels have been implicated as transducers of thermal stimuli, including TRPV1 and TRPV2, which are activated by heat, and TRPM8, which is activated by cold. Here we demonstrate that another member of the TRP family, TRPV4, previously described as a hypo-osmolarity-activated ion channel, also can be activated by heat. In response to warm temperatures, TRPV4 mediates large inward currents in Xenopus oocytes and both inward currents and calcium influx into human embryonic kidney 293 cells. In both cases these responses are observed at temperatures lower than those required to activate TRPV1 and can be inhibited reversibly by ruthenium red. Heat-evoked TRPV4-mediated responses are greater in hypo-osmotic solutions and reduced in hyperosmotic solutions. Consistent with these functional properties, we observed TRPV4 immunoreactivity in anterior hypothalamic structures involved in temperature sensation and the integration of thermal and osmotic information. Together, these data implicate TRPV4 as a possible transducer of warm stimuli within the hypothalamus.
Figures

Similar articles
-
2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3.J Neurosci. 2004 Jun 2;24(22):5177-82. doi: 10.1523/JNEUROSCI.0934-04.2004. J Neurosci. 2004. PMID: 15175387 Free PMC article.
-
Hypothalamic TRPV4 channels participate in the medial preoptic activation of warmth-defence responses in Wistar male rats.Pflugers Arch. 2019 Sep;471(9):1191-1203. doi: 10.1007/s00424-019-02303-1. Epub 2019 Aug 19. Pflugers Arch. 2019. PMID: 31428866
-
Warm temperatures activate TRPV4 in mouse 308 keratinocytes.J Biol Chem. 2003 Aug 22;278(34):32037-46. doi: 10.1074/jbc.M303251200. Epub 2003 Jun 3. J Biol Chem. 2003. PMID: 12783886
-
TRPV4 calcium entry channel: a paradigm for gating diversity.Am J Physiol Cell Physiol. 2004 Feb;286(2):C195-205. doi: 10.1152/ajpcell.00365.2003. Am J Physiol Cell Physiol. 2004. PMID: 14707014 Review.
-
The TRPV4 channel: structure-function relationship and promiscuous gating behaviour.Pflugers Arch. 2003 Jun;446(3):298-303. doi: 10.1007/s00424-003-1028-9. Epub 2003 Apr 25. Pflugers Arch. 2003. PMID: 12715179 Review.
Cited by
-
The functions of TRPA1 and TRPV1: moving away from sensory nerves.Br J Pharmacol. 2012 May;166(2):510-21. doi: 10.1111/j.1476-5381.2012.01851.x. Br J Pharmacol. 2012. PMID: 22233379 Free PMC article. Review.
-
Deletion of TRPV4 enhances in vitro wound healing of murine esophageal keratinocytes.Sci Rep. 2020 Jul 9;10(1):11349. doi: 10.1038/s41598-020-68269-8. Sci Rep. 2020. PMID: 32647282 Free PMC article.
-
TRPV4 activation prevents lipopolysaccharide-induced painful bladder hypersensitivity in rats by regulating immune pathways.Front Immunol. 2022 Dec 22;13:1080302. doi: 10.3389/fimmu.2022.1080302. eCollection 2022. Front Immunol. 2022. PMID: 36618411 Free PMC article.
-
Vanilloid receptors in hearing: altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of corti.J Neurophysiol. 2003 Jul;90(1):444-55. doi: 10.1152/jn.00919.2002. Epub 2003 Mar 26. J Neurophysiol. 2003. PMID: 12660354 Free PMC article.
-
Understanding inflammatory pain: ion channels contributing to acute and chronic nociception.Pflugers Arch. 2010 Apr;459(5):657-69. doi: 10.1007/s00424-010-0784-6. Epub 2010 Feb 17. Pflugers Arch. 2010. PMID: 20162302 Review.
References
-
- Boulant J. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31:S157–S161. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. - PubMed
-
- Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, Crowther D, Sanseau P, Tate SN. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–174. - PubMed
-
- Hori A, Minato K, Kobayashi S. Warming-activated channels of warm-sensitive neurons in rat hypothalamic slices. Neurosci Lett. 1999;275:93–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
