Infliximab for the treatment of rheumatoid arthritis
- PMID: 12137712
- PMCID: PMC8729322
- DOI: 10.1002/14651858.CD003785
Infliximab for the treatment of rheumatoid arthritis
Abstract
Background: Infliximab is a human murine chimeric anti-tumour necrosis factor alpha monoclonal antibody recently approved for the treatment of refractory RA.
Objectives: To assess the efficacy and safety of infliximab for the treatment of rheumatoid arthritis.
Search strategy: Electronic databases including Biological Abstracts, CINAHL, Current Contents, Dissertation Abstracts, EBM Reviews, HealthSTAR and MEDLINE were searched from 1966 to March 2002. Rheumatoid arthritis was searched as an exploded MESH heading. Infliximab was searched as a text word as it is not currently indexed. The search was not limited by language, year of publication or type of publication. The specific search strategy is shown below.
Selection criteria: All randomized controlled trials comparing infliximab 1, 3, 5 or 10 mg/kg with methotrexate(MTX) to MTX alone, or without MTX to placebo, with a minimum duration of 6 months and at least 2 infusions were eligible.
Data collection and analysis: Data was extracted by 2 independent reviewers and the methodological quality of the trials was assessed using a validated assessment tool scale. Outcome variables included the ACR core set of disease activity measures for RA clinical trials and radiographic outcome data. Withdrawals and toxicity were also included. End of trial results were pooled. Continuous data were pooled using weighted mean differences and dichotomous data using relative risks.
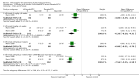
Main results: Two trials with a total of 529 patients met the inclusion criteria. Patients fulfilling the American Rheumatism Association 1987 RA diagnostic criteria were randomized to receive either infliximab 1mg/kg (with and without MTX), 3mg/kg(with and without MTX), 10mg/kg of infliximab (with and without MTX) or placebo infusion plus MTX. Infusions were given every 4 or 8 weeks. After 6 months ACR 20, ACR 50 and ACR 70 response rates were significantly improved in all infliximab doses compared to control. The number needed to treat with infliximab to achieve an ACR 20, 50 or 70 response in patients with refractory RA under specialist care ranged from 2.9 to 3.3 for ACR 20, 3.6 to 4.8 for ACR 50 and 5.9 to 12.5 for ACR 70 depending on the dose (3mg/kg or 10mg/kg given either every 4 or 8 weeks). Total withdrawals and withdrawals due to lack of efficacy were lower for all doses of infliximab versus controls. Withdrawals for adverse events and withdrawals for other reasons were not statistically significantly different for those receiving infliximab from control.
Reviewer's conclusions: Treatment with infliximab for 6 and 12 months significantly reduces RA disease activity and appeared to have an acceptable safety profile in these trials. Total radiographic scores improved, fewer patients showed radiographic progression, and more patients showed radiographic improvement with infliximab treatment at 12 months compared to controls. However, only 2 trials met the inclusion criteria, and these results are largely driven by the largest trial. The available efficacy and toxicity data is relatively short-term (6-12 months). In order to detect rare events that may be associated with infliximab, larger and longer term studies are required.
Conflict of interest statement
None known
Figures








































Similar articles
-
Methotrexate for treating rheumatoid arthritis.Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD000957. doi: 10.1002/14651858.CD000957.pub2. Cochrane Database Syst Rev. 2014. PMID: 24916606 Free PMC article. Review.
-
Adalimumab for treating rheumatoid arthritis.Cochrane Database Syst Rev. 2005 Jul 20;(3):CD005113. doi: 10.1002/14651858.CD005113.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034967 Review.
-
Biologics or tofacitinib for people with rheumatoid arthritis naive to methotrexate: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2017 May 8;5(5):CD012657. doi: 10.1002/14651858.CD012657. Cochrane Database Syst Rev. 2017. PMID: 28481462 Free PMC article. Review.
-
Etanercept for the treatment of rheumatoid arthritis.Cochrane Database Syst Rev. 2003;(4):CD004525. doi: 10.1002/14651858.CD004525. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2013 May 31;(5):CD004525. doi: 10.1002/14651858.CD004525.pub2 PMID: 14584021 Updated. Review.
-
Rituximab for rheumatoid arthritis.Cochrane Database Syst Rev. 2015 Jan 20;1(1):CD007356. doi: 10.1002/14651858.CD007356.pub2. Cochrane Database Syst Rev. 2015. PMID: 25603545 Free PMC article. Review.
Cited by
-
Obesity and Breast Cancer: A Case of Inflamed Adipose Tissue.Cancers (Basel). 2020 Jun 25;12(6):1686. doi: 10.3390/cancers12061686. Cancers (Basel). 2020. PMID: 32630445 Free PMC article. Review.
-
Abatacept for rheumatoid arthritis.Cochrane Database Syst Rev. 2009 Oct 7;2009(4):CD007277. doi: 10.1002/14651858.CD007277.pub2. Cochrane Database Syst Rev. 2009. PMID: 19821401 Free PMC article. Review.
-
A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview.CMAJ. 2009 Nov 24;181(11):787-96. doi: 10.1503/cmaj.091391. Epub 2009 Nov 2. CMAJ. 2009. PMID: 19884297 Free PMC article.
-
Adverse effects of biologics: a network meta-analysis and Cochrane overview.Cochrane Database Syst Rev. 2011 Feb 16;2011(2):CD008794. doi: 10.1002/14651858.CD008794.pub2. Cochrane Database Syst Rev. 2011. PMID: 21328309 Free PMC article. Review.
-
The Comparative Safety of TNF Inhibitors in Ankylosing Spondylitis-a Meta-Analysis Update of 14 Randomized Controlled Trials.Clin Rev Allergy Immunol. 2018 Apr;54(2):234-243. doi: 10.1007/s12016-017-8623-6. Clin Rev Allergy Immunol. 2018. PMID: 28717941
References
References to studies included in this review
Lipsky 2000 {published and unpublished data}
-
- Lipsky PE, Heijde DMFM, Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritis. The New England Journal of Medicine 2000;343:1594‐1602. - PubMed
Maini 1998 {published and unpublished data}
-
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, MacFarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrextae in rheumatoid arthritis. Arthritis and Rheumatism 1998;41(9):1552‐1563. - PubMed
Maini 1999 {published and unpublished data}
-
- Maini R, Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet 1999;354:1932‐1939. - PubMed
References to studies excluded from this review
Brennan 1997 {published data only}
-
- Brennan FM, Browne KA, Green PA, Jaspar JM, Maini RN, Feldmann M. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti‐tumour necrosis factor‐alpha (cA2) therapy. British Journal of Rheumatology 1997;36:643‐650. - PubMed
Elliott 1993 {published data only}
-
- Elliott MJ, Maini RN, Feldmann M, Long‐Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J, Woody JN. Treatment of rheumatoid arthritis with chimeric monoclonal antibody to tumour necrosis factor alpha. Arthritis and Rheumatism 1993;36(12):1681‐1690. - PubMed
Elliott 1994 {published data only}
-
- Elliott MJ, Maini RN, Feldmann M, Long‐Fox A, Charles P, Bijl H, Woody JN. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 1994;344:1125‐1127. - PubMed
Elliott 1994(RCT) {published data only}
-
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, MacFarlane JD, Bijl H, Woody JN. Randomized double‐blind comparision of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 1994;344:1105‐1110. - PubMed
Elliott 1997 {published data only}
-
- Elliott MJ, Woo P, Charles P, Long‐Fox A, Woody JN, Maini RN. Suppression of fever and the acute‐phase response in a patient with juvenile chronic arthritis treated with monoclonal antibody to tumour necrosis factor‐ alpha (cA2). British Journal of Rheumatology 1997;36:589‐593. - PubMed
Kalden‐Nemeth 1997 {published data only}
-
- Kalden‐Nemeth D, Grebmeiser J, Antoni C, Manger B, Wolf F, Kalden JR. NMR monitoring of rheumatoid arthritis patients receiving anti‐TNF alpha monoclonal antibody therapy. Rheumatology International 1997;16:249‐255. - PubMed
Kavanaugh 2000 {published data only}
-
- Kavanaugh A, Clair EW, McCune WJ, Braakman T, Lipsky P. Chimeric anti‐tumour necrosis factor‐alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. Journal of Rheumatology 2000;27:841‐850. - PubMed
Oshima 1999 {published data only}
-
- Oshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Ishida H, Shimizu M, Suemura M, McCloskey R, Kishimoto T. Long‐term floow‐up of the changes in circulating cytokines, soluble cytokine receptors, and white blood cell subset counts in patients with rheumatoid arthritis (RA) after monoclonal anti‐TNF alpha therapy. journal of Clinical Immunology 1999;19:305‐313. - PubMed
Perkins 1998 {published data only}
-
- Perkins DJ, Clair EW, Misukonis MA, Weinberg JB. Reduction of NOS2 overexpression in rheumatoid arthritis patients treated with anti‐tumour necrosis factor alpha monoclonal antibody (cA2). Arthritis and Rheumatism 1998;41(12):2205‐2210. - PubMed
Additional references
Arnett 1988
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31:315‐24. - PubMed
Boers 1994
-
- Boers M, Tugwell P, Felson DT. World health organization and international league of associations for rheumatology core endpoints for symptom modifying antirheumatic drugs in rheumatoid arthritis clinical trials. J Rheumatol 1994;21:86‐9. - PubMed
Felson 1993
-
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. The American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1993;36:729‐40. - PubMed
Felson 1995
-
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38:727‐35. - PubMed
Jadad 1996
-
- Jadad A, Moore A, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Keane 2001
-
- Keane J, Gershon S, Wise RP, Mirabile‐Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha‐neutralizing agent. N Engl J Med 2001;345(15):1098‐1104. - PubMed
OMERACT 1993
-
- OMERACT. Conference on Outcome Measures in Rheumatoid Arthritis Clinical Trials. J Rheumatol 1993;20:526‐91. - PubMed
Pincus 1999
-
- Pincus T, Stein CM. ACR20: clinical or statistical significance?. Arthritis Rheum 1999;42(8):1572‐6. - PubMed
Product Label 2001
-
- [Remicade Product Label]. 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

