Epidermal growth factor receptor dependence of radiation-induced transcription factor activation in human breast carcinoma cells
- PMID: 12134064
- PMCID: PMC117308
- DOI: 10.1091/mbc.01-12-0572
Epidermal growth factor receptor dependence of radiation-induced transcription factor activation in human breast carcinoma cells
Abstract
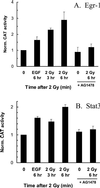
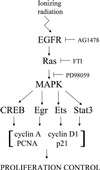
Ionizing radiation (1-5 Gy) activates the epidermal growth factor receptor (EGFR), a major effector of the p42/44 mitogen-activated protein kinase (MAPK) pathway. MAPK and its downstream effector, p90 ribosomal S6 kinase (p90RSK), phosphorylate transcription factors involved in cell proliferation. To establish the role of the EGFR/MAPK pathway in radiation-induced transcription factor activation, MDA-MB-231 human breast carcinoma cells were examined using specific inhibitors of signaling pathways. Gel-shift analysis revealed three different profile groups: 1) transcription factors that responded to both radiation (2 Gy) and epidermal growth factor (EGF) (CREB, Egr, Ets, and Stat3); 2) factors that responded to radiation, but not EGF (C/EBP and Stat1); and 3) those that did not respond significantly to either radiation or EGF (AP-1 and Myc). Within groups 1 and 2, a two- to fivefold maximum stimulation of binding activity was observed at 30-60 min after irradiation. Interestingly, only transcription factors that responded to EGF had radiation responses significantly inhibited by the EGFR tyrosine kinase inhibitor, AG1478; these responses were also abrogated by farnesyltransferase inhibitor (FTI) or PD98059, inhibitors of Ras and MEK1/2, respectively. Moreover, radiation-induced increases in CREB and p90RSK phosphorylation and activation of Stat3 and Egr-1 reporter constructs by radiation were all abolished by AG1478. These data demonstrate a distinct radiation response profile at the transcriptional level that is dependent on enhanced EGFR/Ras/MAPK signaling.
Figures








Similar articles
-
The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner.Cancer Res. 2002 Aug 1;62(15):4307-15. Cancer Res. 2002. PMID: 12154034
-
Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells.Oncogene. 1999 Aug 19;18(33):4756-66. doi: 10.1038/sj.onc.1202849. Oncogene. 1999. PMID: 10467423
-
Increased expression of epidermal growth factor receptor induces sequestration of extracellular signal-related kinases and selective attenuation of specific epidermal growth factor-mediated signal transduction pathways.Mol Cancer Res. 2003 Jan;1(3):219-33. Mol Cancer Res. 2003. PMID: 12556561
-
Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death.Mol Biol Cell. 1999 Aug;10(8):2493-506. doi: 10.1091/mbc.10.8.2493. Mol Biol Cell. 1999. PMID: 10436007 Free PMC article.
-
Steering tumor progression through the transcriptional response to growth factors and stroma.FEBS Lett. 2014 Aug 1;588(15):2407-14. doi: 10.1016/j.febslet.2014.05.036. Epub 2014 May 27. FEBS Lett. 2014. PMID: 24873881 Free PMC article. Review.
Cited by
-
Expanding the therapeutic repertoire of epidermal growth factor receptor blockade: radiosensitization.Breast Cancer Res. 2003;5(3):126-9. doi: 10.1186/bcr584. Epub 2003 Feb 20. Breast Cancer Res. 2003. PMID: 12793892 Free PMC article. Review.
-
Targeting DNA Double-Strand Break Repair Pathways to Improve Radiotherapy Response.Genes (Basel). 2019 Jan 4;10(1):25. doi: 10.3390/genes10010025. Genes (Basel). 2019. PMID: 30621219 Free PMC article. Review.
-
Nitration of the tumor suppressor protein p53 at tyrosine 327 promotes p53 oligomerization and activation.Biochemistry. 2010 Jun 29;49(25):5331-9. doi: 10.1021/bi100564w. Biochemistry. 2010. PMID: 20499882 Free PMC article.
-
STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells.Proc Natl Acad Sci U S A. 2004 Feb 10;101(6):1714-9. doi: 10.1073/pnas.0308102100. Epub 2004 Jan 30. Proc Natl Acad Sci U S A. 2004. PMID: 14755057 Free PMC article.
-
Augmentation of radiation response by panitumumab in models of upper aerodigestive tract cancer.Int J Radiat Oncol Biol Phys. 2008 Oct 1;72(2):534-42. doi: 10.1016/j.ijrobp.2008.06.1490. Int J Radiat Oncol Biol Phys. 2008. PMID: 18793955 Free PMC article.
References
-
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
-
- Andrisani OM. CREB-mediated transcriptional control. Crit Rev Eukaryot Gene Expr. 1999;9:19–32. - PubMed
-
- Beier F, Taylor AC, LuValle P. The Raf-1/MEK/ERK pathway regulates the expression of the p21(Cip1/Waf1) gene in chondrocytes. J Biol Chem. 1999;274:30273–30279. - PubMed
-
- Beier F, Taylor AC, LuValle P. Activating transcription factor 2 is necessary for maximal activity and serum induction of the cyclin A promoter in chondrocytes. J Biol Chem. 2000;275:12948–12953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

