Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation
- PMID: 12110596
- PMCID: PMC126103
- DOI: 10.1093/emboj/cdf353
Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation
Abstract
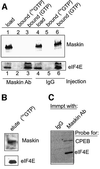
Cytoplasmic polyadenylation stimulates the translation of several dormant mRNAs during oocyte maturation in Xenopus. Polyadenylation is regulated by the cytoplasmic polyadenylation element (CPE), a cis-acting element in the 3'-untranslated region of responding mRNAs, and its associated factor CPEB. CPEB also binds maskin, a protein that in turn interacts with eIF4E, the cap-binding factor. Here, we report that based on antibody and mRNA reporter injection assays, maskin prevents oocyte maturation and the translation of the CPE-containing cyclin B1 mRNA by blocking the association of eIF4G with eIF4E. Dissociation of the maskin-eIF4E complex is essential for cyclin B1 mRNA translational activation, and requires not only cytoplasmic polyadenylation, but also the poly(A)-binding protein. These results suggest a molecular mechanism by which CPE- containing mRNA is activated in early development.
Figures






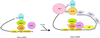
Similar articles
-
The control of cyclin B1 mRNA translation during mouse oocyte maturation.Dev Biol. 2000 May 1;221(1):1-9. doi: 10.1006/dbio.2000.9669. Dev Biol. 2000. PMID: 10772787
-
Translational control of cyclin B1 mRNA during meiotic maturation: coordinated repression and cytoplasmic polyadenylation.Dev Biol. 2000 Apr 1;220(1):97-109. doi: 10.1006/dbio.2000.9613. Dev Biol. 2000. PMID: 10720434
-
CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus.EMBO J. 1996 May 15;15(10):2582-92. EMBO J. 1996. PMID: 8665866 Free PMC article.
-
Translational regulation during oogenesis and early development: the cap-poly(A) tail relationship.C R Biol. 2005 Oct-Nov;328(10-11):863-81. doi: 10.1016/j.crvi.2005.05.006. Epub 2005 Jun 8. C R Biol. 2005. PMID: 16286077 Review.
-
Cytoplasmic mRNA polyadenylation and translation assays.Methods Mol Biol. 2006;322:183-98. doi: 10.1007/978-1-59745-000-3_13. Methods Mol Biol. 2006. PMID: 16739724 Review.
Cited by
-
Poly(A) binding proteins: are they all created equal?Wiley Interdiscip Rev RNA. 2013 Mar-Apr;4(2):167-79. doi: 10.1002/wrna.1151. Epub 2012 Dec 13. Wiley Interdiscip Rev RNA. 2013. PMID: 23424172 Free PMC article. Review.
-
Specificity factors in cytoplasmic polyadenylation.Wiley Interdiscip Rev RNA. 2013 Jul-Aug;4(4):437-61. doi: 10.1002/wrna.1171. Wiley Interdiscip Rev RNA. 2013. PMID: 23776146 Free PMC article. Review.
-
Ultrasensitive Ribo-seq reveals translational landscapes during mammalian oocyte-to-embryo transition and pre-implantation development.Nat Cell Biol. 2022 Jun;24(6):968-980. doi: 10.1038/s41556-022-00928-6. Epub 2022 Jun 13. Nat Cell Biol. 2022. PMID: 35697785
-
Trading translation with RNA-binding proteins.RNA. 2008 Mar;14(3):404-9. doi: 10.1261/rna.848208. Epub 2008 Jan 22. RNA. 2008. PMID: 18212021 Free PMC article. Review.
-
Alternative splicing of the mouse embryonic poly(A) binding protein (Epab) mRNA is regulated by an exonic splicing enhancer: a model for post-transcriptional control of gene expression in the oocyte.Mol Hum Reprod. 2008 Jul;14(7):393-8. doi: 10.1093/molehr/gan028. Epub 2008 May 20. Mol Hum Reprod. 2008. PMID: 18492745 Free PMC article.
References
-
- Bilger A., Fox,C.A., Wahle,E. and Wickens,M. (1994) Nuclear poly adenylation factors recognize cytoplasmic polyadenylation elements. Genes Dev., 8, 1106–1116. - PubMed
-
- Cornelis S., Bruynooghe,Y., Denecker,G., Van Huffel,S., Tinton,S. and Beyaert,R. (2000) Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol. Cell, 5, 597–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

