A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer
- PMID: 12107836
- PMCID: PMC2376102
- DOI: 10.1038/sj.bjc.6600446
A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer
Abstract
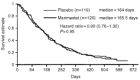
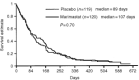
Pancreatic cancer is the fifth most common cause of cancer death in the western world and the prognosis for unresectable disease remains poor. Recent advances in conventional chemotherapy and the development of novel 'molecular' treatment strategies with different toxicity profiles warrant investigation as combination treatment strategies. This randomised study in pancreatic cancer compares marimastat (orally administered matrix metalloproteinase inhibitor) in combination with gemcitabine to gemcitabine alone. Two hundred and thirty-nine patients with unresectable pancreatic cancer were randomised to receive gemcitabine (1000 mg m(-2)) in combination with either marimastat or placebo. The primary end-point was survival. Objective tumour response and duration of response, time to treatment failure and disease progression, quality of life and safety were also assessed. There was no significant difference in survival between gemcitabine and marimastat and gemcitabine and placebo (P=0.95 log-rank test). Median survival times were 165.5 and 164 days and 1-year survival was 18% and 17% respectively. There were no significant differences in overall response rates (11 and 16% respectively), progression-free survival (P=0.68 log-rank test) or time to treatment failure (P=0.70 log-rank test) between the treatment arms. The gemcitabine and marimastat combination was well tolerated with only 2.5% of patients withdrawn due to presumed marimastat toxicity. Grade 3 or 4 musculoskeletal toxicities were reported in only 4% of the marimastat treated patients, although 59% of marimastat treated patients reported some musculoskeletal events. The results of this study provide no evidence to support a combination of marimastat with gemcitabine in patients with advanced pancreatic cancer. The combination of marimastat with gemcitabine was well tolerated. Further studies of marimastat as a maintenance treatment following a response or stable disease on gemcitabine may be justified.
Figures
Similar articles
-
Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial.J Clin Oncol. 2001 Aug 1;19(15):3447-55. doi: 10.1200/JCO.2001.19.15.3447. J Clin Oncol. 2001. PMID: 11481349 Clinical Trial.
-
BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer.Ann Oncol. 2012 Nov;23(11):2799-2805. doi: 10.1093/annonc/mds135. Epub 2012 Jul 5. Ann Oncol. 2012. PMID: 22771827 Clinical Trial.
-
A phase II trial of marimastat in advanced pancreatic cancer.Br J Cancer. 2001 Dec 14;85(12):1865-70. doi: 10.1054/bjoc.2001.2168. Br J Cancer. 2001. PMID: 11747327 Free PMC article. Clinical Trial.
-
Gemcitabine-based combination therapy compared with gemcitabine alone for advanced pancreatic cancer: a meta-analysis of nine randomized controlled trials.Hepatobiliary Pancreat Dis Int. 2017 Jun;16(3):236-244. doi: 10.1016/s1499-3872(17)60022-5. Hepatobiliary Pancreat Dis Int. 2017. PMID: 28603091 Review.
-
Gemcitabine-based chemotherapy for advanced biliary tract carcinomas.Cochrane Database Syst Rev. 2018 Apr 6;4(4):CD011746. doi: 10.1002/14651858.CD011746.pub2. Cochrane Database Syst Rev. 2018. PMID: 29624208 Free PMC article. Review.
Cited by
-
Silencing miR-202-3p increases MMP-1 and promotes a brain invasive phenotype in metastatic breast cancer cells.PLoS One. 2020 Oct 1;15(10):e0239292. doi: 10.1371/journal.pone.0239292. eCollection 2020. PLoS One. 2020. PMID: 33002044 Free PMC article.
-
Quality of life of patients with metastatic pancreatic adenocarcinoma initiating first-line chemotherapy in routine practice.BMC Palliat Care. 2020 Jul 10;19(1):103. doi: 10.1186/s12904-020-00610-4. BMC Palliat Care. 2020. PMID: 32650765 Free PMC article.
-
Inhibiting the growth of pancreatic adenocarcinoma in vitro and in vivo through targeted treatment with designer gold nanotherapeutics.PLoS One. 2013;8(3):e57522. doi: 10.1371/journal.pone.0057522. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23483913 Free PMC article.
-
Enhancing sorafenib-mediated sensitization to gemcitabine in experimental pancreatic cancer through EMAP II.J Exp Clin Cancer Res. 2013 Mar 6;32(1):12. doi: 10.1186/1756-9966-32-12. J Exp Clin Cancer Res. 2013. PMID: 23497499 Free PMC article.
-
Systemic therapies for pancreatic cancer--the role of pharmacogenetics.Curr Drug Targets. 2012 Jun;13(6):811-28. doi: 10.2174/138945012800564068. Curr Drug Targets. 2012. PMID: 22458528 Free PMC article. Review.
References
-
- BaileyAJParmarMKStephensRJ1998Patient-reported short-term and long-term physical and psychologic symptoms: results of the continuous hyperfractionated accelerated (correction of acclerated) radiotherapy (CHART) randomized trial in non-small-cell lung cancer. CHART Steering Committee J Clin Oncol 1630823093 - PubMed
-
- BramhallSRAllumWHJonesAGAllwoodACumminsCNeoptolemosJP1995Incidence, treatment and survival in 13,560 patients with pancreatic cancer: An epidemiological study in the West Midlands Br J Surg 82111115 - PubMed
-
- BramhallSRHallisseyMTWhitingJScholefieldJTierneyGStuartRCHawkinsREMcCullochPMaughanTBrownPDBailletMFieldingJWL2001aMarimastat as maintenance therapy for patients with advanced gastric and gastro-oesophageal cancer: A randomized trial Br J Cancer(in press)
-
- BramhallSRNeoptolemosJP1997Adjuvant chemotherapy in pancreatic cancer Int J Pancreatol 215963 - PubMed
-
- BramhallSRNeoptolemosJPStampGWLemoineNR1997Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma J Pathol 182347355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical