The nitric oxide synthase inhibitor NG-Nitro-L-arginine increases basal forebrain acetylcholine release during sleep and wakefulness
- PMID: 12097511
- PMCID: PMC6758209
- DOI: 10.1523/JNEUROSCI.22-13-05597.2002
The nitric oxide synthase inhibitor NG-Nitro-L-arginine increases basal forebrain acetylcholine release during sleep and wakefulness
Abstract
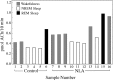
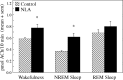
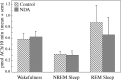
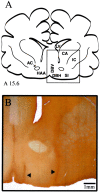
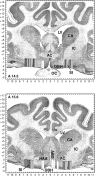
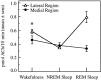
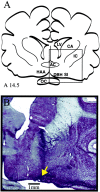
Cholinergic neurotransmission in the basal forebrain changes across the sleep/wake cycle, and considerable data show cortical activation by ACh originating from basal forebrain neurons. These findings have stimulated efforts to elucidate molecular modulators of ACh release within the basal forebrain. Basal forebrain cholinergic neurons contain nitric oxide synthase (NOS), the enzyme that produces the gaseous neuromodulator nitric oxide. This study tested the hypothesis that administration of an NOS inhibitor to the basal forebrain would alter basal forebrain ACh release, sleep, and respiratory rate. Seven cats were instrumented for recording sleep and wakefulness and for in vivo microdialysis and microinjection. Compared with Ringer's solution (control), microdialysis delivery of the NOS inhibitor N(G)-nitro-l-arginine (NLA; 10 mm) increased ACh release during wakefulness (33%), non-rapid eye movement (NREM) sleep (70%), and rapid eye movement (REM) sleep (16%). Mean +/- SEM ACh levels (pmol/10 min) during control and NLA dialysis, respectively, were 0.58 +/- 0.03 and 0.77 +/- 0.06 in wakefulness, 0.36 +/- 0.01 and 0.61 +/- 0.06 in NREM sleep, and 0.68 +/- 0.06 and 0.79 +/- 0.09 in REM sleep. Increases in ACh release were not evoked by dialysis delivery of the less active enantiomer N(G)-nitro-d-arginine. Dialysis administration of NLA did not alter respiratory rate. Sleep-dependent changes in basal forebrain ACh release were localized specifically to lateral basal forebrain regions and did not occur in medial basal forebrain sites. Microinjection of NLA into the lateral basal forebrain did not significantly alter the sleep/wake cycle. In contrast to NLA-induced depression of REM sleep and ACh release in the cat pons, the present results demonstrate that NLA increased ACh release in the cat basal forebrain and had no effect on sleep. The different effects of NLA on ACh release in the cat pons and cat basal forebrain may prove relevant for developing compounds that differentially alter cholinergic neurotransmission in specific brain regions.
Figures
Similar articles
-
Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate.J Neurosci. 1997 Jan 15;17(2):774-85. doi: 10.1523/JNEUROSCI.17-02-00774.1997. J Neurosci. 1997. PMID: 8987799 Free PMC article.
-
Nitric oxide synthase inhibition decreases pontine acetylcholine release.Neuroreport. 1995 Jul 31;6(11):1525-9. doi: 10.1097/00001756-199507310-00015. Neuroreport. 1995. PMID: 7579140
-
Basal forebrain acetylcholine release during REM sleep is significantly greater than during waking.Am J Physiol Regul Integr Comp Physiol. 2001 Feb;280(2):R598-601. doi: 10.1152/ajpregu.2001.280.2.R598. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11208592
-
[Selective stimulations and lesions of the rat brain nuclei as the models for research of the human sleep pathology mechanisms].Glas Srp Akad Nauka Med. 2011;(51):85-97. Glas Srp Akad Nauka Med. 2011. PMID: 22165729 Review. Serbian.
-
Is the EDRF in the cerebral circulation NO? Its release by shear and the dangers in interpreting the effects of NOS inhibitors.Keio J Med. 1998 Sep;47(3):142-9. doi: 10.2302/kjm.47.142. Keio J Med. 1998. PMID: 9785759 Review.
Cited by
-
Brainstem stimulation increases functional connectivity of basal forebrain-paralimbic network in isoflurane-anesthetized rats.Brain Connect. 2014 Sep;4(7):523-34. doi: 10.1089/brain.2014.0254. Brain Connect. 2014. PMID: 25090190 Free PMC article.
-
GABA-to-ACh ratio in basal forebrain and cerebral cortex varies significantly during sleep.Sleep. 2012 Oct 1;35(10):1325-34. doi: 10.5665/sleep.2106. Sleep. 2012. PMID: 23024430 Free PMC article.
-
Nitric oxide-mediated cortical activation: a diffuse wake-up system.J Neurosci. 2003 May 15;23(10):4299-307. doi: 10.1523/JNEUROSCI.23-10-04299.2003. J Neurosci. 2003. PMID: 12764118 Free PMC article.
-
Control of sleep and wakefulness.Physiol Rev. 2012 Jul;92(3):1087-187. doi: 10.1152/physrev.00032.2011. Physiol Rev. 2012. PMID: 22811426 Free PMC article. Review.
References
-
- Baghdoyan HA, Lydic R. Neurotransmitters and neuromodulators regulating sleep. In: Bazil C, Malow B, Sammaritano M, editors. Sleep and epilepsy: the clinical spectrum. Elsevier Science; New York: 2002. pp. 17–44.
-
- Baghdoyan HA, Lydic R, Fleegal MA. M2 muscarinic autoreceptors modulate acetylcholine release in the medial pontine reticular formation. J Pharmacol Exp Ther. 1998;286:1446–1452. - PubMed
-
- Benevento LA, McCleary LB. An immunocytochemical method for marking microelectrode tracks following single-unit recordings in long surviving, awake monkeys. J Neurosci Methods. 1992;41:199–204. - PubMed
-
- Berman AL, Jones EG. The thalamus and basal telencephalon of the cat. University of Wisconsin; Madison: 1982.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
